|
Last Update:
Thursday November 22, 2018
|
| [Home] |
|
Volume 17 Pages 1 - 58 (April 2000) Citation: Green, R. (2000) Sexual Differences in the Behaviour of Young Otters (Lutra lutra) IUCN Otter Spec. Group Bull. 17 (1): 20 - 30 Sexual Differences in the Behaviour of Young Otters (Lutra lutra) Rosemary Green Vincent Wildlife Trust, Barjarg, Barrhill, Girvan, Ayrshire KA26 ORB, United Kingdom (received 27th November 1999, accepted 19 December, 1999)
INTRODUCTION Differences in the behaviour of young animals, which it was supposed were gender based, had been noted over 15 years of caring for otters. When the opportunity of studying two same sex pairs arose, detailed observations were made to compare and quantify behaviour. These observations are presented, despite the small sample and unsystematic nature of the data, in the hope that they will stimulate more work by organisations with captive otters. ANIMALS, MATERIALS AND METHODS Animals observed were a male from Northern Ireland, (estimated birth date 14.6.98) and three siblings from Unst, Shetland (estimated birth date 23.7.98). The siblings were reared together until comfort sucking of the male necessitated his removal to more congenial company. The two pairs, males of different ages and backgrounds and the two sisters of the Unst male were observed for 105 days from 15.10.98, when they were aged 4 -5 months, to 28.2.99. Days were divided into 30 min. slots from 06:00 to 24:00 and behavioural observations made each day. Differences in the position of bedding and feeding bowls noted led to recording the location of these objects for part of the period. The animals were housed in identical indoor pens, side by side, with a gutter for sprainting and cleaning, two beds at the end opposite the door and a grille which allowed the pairs to see and touch each other. The grille did not quite reach the floor, allowing some objects to be pulled under it. Each pair had an empty pen on the other side. Pens were cleaned in early morning and otters fed then and in late afternoon. Other work commitments made regular, systematic observation impossible so some time periods, particularly feeding and pen cleaning times, were better covered than others. Pen walls were 1.5m high, allowing observation from the corridor without disturbance. Each observation lasted five minutes and each 30 minute slot had at least 40 observations. The position of spraint in the pens was noted at cleaning time and on 50 occasions the spraint was weighed. RESULTS As observations were not systematic it was only possible to show frequencies of activity, not percentage of time spent on each. Table 1 shows numbers and percentages of each activity recorded for each pair.
1) Spraints and sprainting Sprainting accounted for only 3.7% (n=190/5136) of activity, but was observed over 7 times more frequently by males than by females (166:24). Some of this difference was because the males deliberately positioned themselves over their spraint site, lifted their tails high and deposited spraint, but the females usually sprainted while performing other activities. In addition to differences in frequency of sprainting there were differences in timing and position of spraint in the pens. The males had one spraint site in the gutter adjoining the grille giving onto the females' pen, where almost all spraint was deposited; the only exceptions were objects introduced into the pen which were marked with spraint on arrival. The single spraint heap was kept tidy by careful positioning before sprainting and the males avoided walking over it. In contrast, the females sprainted anywhere in their pen, even in beds, food or water bowls. They sprainted while walking about, trailing spraint over the floor, and spread it by walking or dragging bedding through it. After cleaning time the males promptly renewed their sign heap, never taking more than 5 minutes to produce the first spraints and sometimes snapping at the cleaner's feet in order to do so. The females did not respond to pen cleaning, taking 30 -120 minutes before sprainting was observed, always after eating their first meal. As the volume of the males' spraint heap remained constant, despite differences in the solid matter in the food provided, the wet weight of spraint was recorded on 50 occasions. It proved impossible to collect all female spraint most days, so their results are fewer and less reliable.
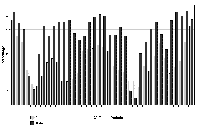
2) Sleeping/resting Sleeping or resting was the most frequently observed occupation (2000/5136 = 38.9%), but was observed over 50% more by males (1212/2554 = 47.5%) than by females (788/2582 =30.5%). There were also differences in the location and timing of the activity. The males always slept together in the same bed and rarely moved their bedding from it, while the females moved their bedding frequently, slept in either bed or elsewhere, with or without bedding, alone or together. In all time slots except 7:00-8:30, 17:30-18:30 the percentage of times at which the males were recorded sleeping or resting exceeded that of the females; significantly these periods were the usual feeding times. Apart from feeding times, the males lay in bed for 41-74% of total activity recorded in each time slot. The females were more active than the males in all time slots, except those covering feeding times, with sleeping accounting for 19- 68% of recorded activity . Besides the greater activity shown around feeding times, the females showed a pattern of rest and activity throughout the day, with rests after breakfast, between 13:00 and 15:30 and again after supper. Females slept for the highest percentage of recorded activity late at night and early in the morning. In the few observations between midnight and 06:00 all otters slept. These results are shown in Fig. 1.
3) Playing Playing was the second most frequently observed activity (986/5136 =19.2%). However there were marked differences between the sexes, with playing being the most often recorded female activity (924/2582 =35.8%), but second least frequent for males (62/2554 =2.4%). Male play was difficult to record as it was either rough, shading into fighting, or gentle nuzzling and wrestling while lying in bed. Female play was active, vocal and prolonged, using bedding, water, bowls and food in games. The females interacted playfully with the carer at cleaning or feeding times, standing on their hind legs in the stream of water, trying to catch it with paws and teeth, or running off with brushes and cleaning materials. 4) Calling for Food, Eating, Grooming and Fighting These activities are grouped as they were related. Calls for food were addressed to the observer, consisting of whistles, chitters and moans, while standing or jumping at the pen door. Males called for food on 104 occasions (4.1% of their total recorded activity). As feeding time approached entreaties became more insistent, but the arrival of the observer was greeted by hopeful calls in all time slots. The females called less often (16/2582 =0.6%) and only immediately before feeding time.
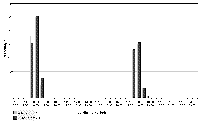
Eating was the third most frequently recorded activity (750/5136 =14.6%) with similar percentages recorded for both sexes (males - 414/2554 =16.2%; females -336/2582 =13%). Differences in the timing and manner of eating were shown by the two pairs. Males were recorded eating only in time slots covering feeding times, with all food, including most fish heads, eaten quickly; see Fig. 2. The females did not eat so avidly, making their food last longer, although most eating was recorded immediately after feeding time, see Fig.3. They often left the heads, which were eagerly seized by the males if within reach. The males became agitated when food arrived, sometimes fighting or biting the carer, so they were thrown food before entering the pen. The Irish male never let his companion keep the first fish, if he happened to catch it. Fighting between the males occurred on 78 occasions (3.1% of total activity), but was recorded only in the time slots covering feeding time and always associated with squabbles over food. Fig. 2 shows the association of feeding and fighting among the males. To minimise fighting the males were given equal numbers and sizes of fish at each meal. In contrast the females showed as much interest in the carer bringing the food as in the food itself. They fought on only four occasions, not linked to food. The females would sometimes share a large fish between them without dispute.
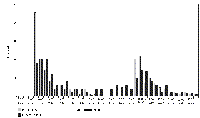
Grooming showing marked sexual, differences; it was the third most frequently observed female activity (472/2582 =18.3%), but was not seen at all by the males. The only time slot in which female grooming was not observed was 14:30-15:00, but although some grooming took place throughout the day the activity peaked after meal times: see Fig. 3. 5) Watching the Neighbours This was the second most often recorded activity of the males (518/2554 = 20.3%); most of the time they were out of bed they were stationed at the grille between the pens watching the females, and whenever possible, taking objects from them. Positions of bedding and feeding bowls of both pairs were recorded on 198 occasions, see Table 2. The males managed to acquire the females' feeding bowl and bedding on many occasions, helped by the females' habit of dragging their bedding about and rolling their bowl around in play. In one incident they broke the females' water bowl trying to pull too large an object under the grille and on several noisy occasions they managed to catch hold of a female's tail or leg and tried to pull her into their pen. The females largely ignored their neighbours, showing interest only if something noisy or unusual was happening, being recorded watching them on only 18 (0.7% of total activity) occasions. DISCUSSION a) Activity patterns There were both quantitative and qualitative differences in the activity of young male and female otters. Both sexes spent much of their time inactive, but the males were less active overall, sleeping or resting more often and longer, playing less vigorously, not grooming at all and spending much time standing quietly watching the females. ROSOUX (1995) found that an adult male otter, radio tracked for 66 days, spent only 30% of its time active. Available evidence suggests that these young otters all slept between midnight and 06:00 reducing further their daily activity. Most studies of wild animals indicate a predominantly nocturnal activity pattern (GREEN et al.,1984; JEFFERIES et al.,1986; ROSOUX, 1995; KRANZ, 1995; SJOASEN, 1997; VOGEL, 1997, 1998), but some studies in Scotland and Norway (WATSON, 1978; LIGHTFOOT, 1985; KRUUK, 1995; TWELVES, unpubl.data; own unpubl. data) report a partially or wholly diurnal pattern of activity. A diurnal activity pattern maybe the norm for Shetland otters, as described by KRUUK (1995) and have been established very early in the life of the siblings from Unst, or it may have been simply a response to the diurnal rhythm of their care. Radio tracking studies of wild otters all show a cycle of activity interspersed by periods of rest. This behaviour seems to be established early in life, as the female pair showed a pattern of rests after meals and in early afternoon from 4 months. Overall the females were active together more often, but the males were together more as they always slept together. Playfulness, popularly considered characteristic of young otters, was shown, in this study, only by the females. With their habit of playing with food and water, either from their drinking bowl or during pen cleaning, and their messy sprainting behaviour, the females' pen was frequently wet and dirty. Because they dragged it around the pen, their bedding also got wet and dirty as soon as it was changed. This was in contrast to the dry, clean pen and bedding of the males; they sprainted in one place, ate their food quickly and completely and did not play with their bedding, bowls, or water. With their messy pen, habit of playing with food and their boisterous play ,the females got their fur dirtier than the males. Grooming was more necessary for the females than for the males, but it was also a joint activity, with some mutual grooming. NOLET and KRUUK (1989) found that radio tracked otters in Shetland spent 6% of their time grooming and that grooming was directly related to diving, especially in deep water, but not to the length of time in the water. In this study grooming was related to the water games played when the water bowls were refilled at feeding times for the females. b) Relationships and Dominance. Relations between the females were friendly and equable, both during the study and afterwards, but the males had a more competitive relationship. The older Irish male dominated the younger, but bigger, Shetland male. His dominance was shown at feeding time, when he always had the first fish, taking it from his companion if he happened to get it. On other occasions the Irish male stayed in bed and took fish which the Shetland male brought to him. Feeding time was tense, each male ate as much as he could as quickly as possible, in contrast to the females which played with and shared their food over a longer time period. Access to food was one of the few opportunities for establishing the balance of the relationship within the confines of the pen. The Shetland male did not always acquiesce readily to the situation as 78 squabbles over food were observed, but in almost every case the Irish male was able to reinforce his status. This may have been because he was older, had a more dominant personality or because he was the original occupant of the shared pen. He remained dominant until release, although the size difference increased as they grew (5.05: 6.55kg) and the animals were moved into a large outdoor pen. Even male siblings reared showed a dominant/ subordinate relationship; in one case the larger was dominant and in the other both brothers were dominant to an unrelated, larger male of similar age. Studies of wild otters all show that some males have access to the best habitat and breeding opportunities, while others are excluded, being forced to lead a transient life in marginal habitat. KRUUK (1995) witnessed a majority of meetings between male otters ending in fights and SIMPSON (1997) and GREEN and GREEN (in press) both report males with severe injuries or deaths resulting from fights. This study suggests that such male behaviour begins early in life and is not based only on size or strength. The situation for females is less clear cut, some authors (GREEN et al. 1984; KRUUK, 1995) record mutual avoidance between adult females, or friendly interaction (KRUUK, 1995), but SJOASEN (1997) found aggressive female relationships. The females in this study were sisters so their behaviour as juveniles may not be indicative of their relationship as adults. The males were intensely interested in the females, spending much of their time watching them, but were interested in the carer only as a supplier of food, reacting in an aggressive/defensive manner when their pen was entered and defending their dirty bowls and bedding. In contrast the females showed little interest in the males, but were very curious about the world outside their pen and in the carer, who was subject to close examination with nose and paws while in their pen. Care had to be taken when opening either pen door as the males were likely to bite anything entering and the females were likely to rush outside. c) Sprainting. Marked differences between the pairs were shown in sprainting behaviour. The frequency of male sprainting was higher than that of the females, even allowing for under recording of female sprainting because of their less obvious sprainting behaviour. The males sprainted at a single site, the only point in their pen where the females could see and smell their sign heap. The sign heap was renewed as soon as it had been cleared away during pen cleaning and its integrity maintained, whereas the females sprainted wherever they happened to be, often during other activities, disregarding the spraint thereafter. Radio isotope studies (JENKINS 1980; GREEN et al., 1984; TWELVES unpubl.) suggest that males spraint mark more frequently than females. This is borne out by HILLEGAART et al. (1985) who recorded male captives sprainting 7 times per active hour compared with 3 times for females. However KRUUK (1995) found no differences in the frequencies of sprainting, regardless of sex or age. There is debate about the significance of sprainting for wild otters. Some authors follow ERLINGE (1967, 1968, 1985) in assuming that spraints are used to mark territory, but KRUUK (1995) links spraint marking to use of food resources and found no increase in frequency of marking near known range boundaries. Some spraint is clearly left where other otters will find it, as otters go out of their way to examine spraint on traditional spraint sites and to leave their own. Many traditional sites are sheltered and spraint may persist there for up to a year (MASON and MACDONALD, 1986). Captive studies (GORMAN and TROWBRIDGE, 1989; ROZHNOV and ROGOSCHIK, 1994; HEINS, 1996) show that otters can recognise the spraint of known individuals over long time periods. TSCHIRCH et al. (1996) found it possible to determine chemically sex and breeding status of otters depositing spraint, information which is presumably also available to otters. These otters showed similarities with the behaviour of wild otters and other captive studies. The males renewed their sign heap when it was washed away, as wild otters renew signs after a spate (KRANZ, 1995; pers.obs.). The site chosen was their interface with neighbouring otters and new objects entering the pen were marked with spraint, as wild otters mark new territory visited. The males kept the volume and wet weight of spraint produced fairly constant, regardless of the amount of indigestible material in their food. Deposition of gut mucus when a larger number of spraints, than can be produced from the food remains in the gut, is needed is well known from the wild. However, it is more difficult to understand why the males should have produced less spraint than the females when food with a high proportion of indigestible matter was fed., especially as they usually ate more of it. These results suggest that spraint marking has territorial and social significance for male otters from an early age. However the young females appeared to be simply defaecating in the course of their periods of activity, which be a precursor of the temporary spraint marking of resources in use seen by Kruuk in Shetland. The males were unable to expand their territory as the size of the pen was finite, so they sought to increase their sphere of influence by taking items from other otters, but did not take brushes, etc. from the carer, as the females did in play. In conclusion, many of the behaviour patterns of wild otters were seen in these young otters but there were significant differences between males and females. As all of these otters had been abandoned before the usual age at which cubs leave the natal holt and had had no chance to observe or experience such behaviour, it is suggested that these differences are innate. ACKNOWLEDGEMENTS - Grateful thanks to Sandy, Shirley, Sean and Alastair for bearing with my strange behaviour outside their pens for so long. REFERENCES Erlinge, S. (1967). Home range of the
otter (Lutra lutra) in southern Sweden. Oikos 18:
186-209. RESÚMEN: Diferencias sexuales en el comportatniento de nutrias (Lutra lutra) jóvenes Para estudiar diferencias vinculadas al sexo en el comportamiento de nutrias jóvenes se observó el comportamiento en cautiverio de cuatro nutrias, 2 machos con distintas edades y antecedentes y 2 hermanas de la misma edad y origen. Los animales de cada sexo fueron colocados juntos en 2 recintos iguales separados por una reja que permitia que los animales se vieran y tocaran. El marcaje representó sólo el 3.7% de la actividad de las nutrias, pero fue 7 veces más frecuente en los machos que en las hembras. Los machos depositaban deliberadamente las fecas en un único sitio fijo de marcaje (y en los objetos que se introducian al recinto), mientras que las hembras las depositaban en cualquier lado mientras realizaban otras actividades. Los machos renovaban el sitio de marcaje inmediatamente después (en un tiempo no mayor a los 5 minutos) que este era limpiado por el cuidador, mientras que las hembras no lo hacian hasta después de la comida. El descanso fue la actividad más frecuentemente observada (38.9%), pero fue observada más frecuentemente en machos (47.5%) que en hembras (30.5%). Los machos siempre durmieron juntos en el mismo lecho, y raramente
movieron el mismo, mientras que las hembras cambiaron frecuentemente el
sitio de descanso, durmiendo juntas o separadas en cualquiera de los 2
lechos de su encierro, o en algún otro lado. Durante las pocas
observaciones realizadas entre la medianoche y las 6 AM, todas las
nutrias dormian. El juego fue la segunda actividad más frecuente
observada (19.2%), siendo la actividad más común en las hembras
(35.8%), y la segunda menos frecuente en machos (2.4%). Comer fue la
tercer actividad más común registrada (14.6%), con porcentajes
similares en los 2 sexos (machos 16.2%, hembras 13%). Los machos só1o
fueron registrados comiendo durante el periodo inmediatamente posterior
a que se les entregaba la comida, ingiriéndola rápidamente, incluyendo
la cabeza de los pescados. Las hembras hacían durar más la comida,
aunque la mayoría de la de los registros de alimentación se realizaron
inmediatamente después de que se les entregaba la misma. Comúnmente
dejaban las cabezas, que eran ávidamente consumidas por los machos si
eran capaces de alcanzarlas. El macho más adulto nunca dejó a su
compañero quedarse con el primer pescado, aún cuando este lo agarrara.
Se registraron peleas 78 veces entre los machos (3.1% de la actividad
total), y só1o durante periodos de alimentación y vinculadas con la
comida. El mayor dominaba al más joven aún cuanto este último era de
mayor tamaño. Durante el acceso a la comida era uno de los pocos
mementos en los que se podía establecer este balance en la relación.
Só1o se registraron 4 peleas entre las hembras, ninguna vinculada a la
comida. Las hembras compartían en algunos casos un pescado grande entre
las 2 sin ninguna disputa. El grooming fue la tercera actividad más
frecuente en hembras (18.3%), y no se registró en machos. Dado el
comportamiento desprolijo de marcado en las hembras y sus hábitos de
jugar con la comida y el agua, éstas conseguían ensuciar más su
pelaje que los machos, por lo que el grooming era más necesario en
éstas que en aqueilos. Observar a los vecinos fue la segunda actividad
más frecuente en machos (20.3%). Observaban a las hembras mayormente
junto a la reja que separaba los encierros, y cuando era posible tomaban
objetos de las mismas. Las hembras ignoraban mayormente a sus vecinos,
mostrando interés sólo si ocurría algo inusual. Sólo se las
registró observando a los machos en 18 ocasiones (0.7%). Estos animales
mostraron similitudes en el comportamiento con el registrado en animales
en libertad y en otros estudios en cautiverio. Los machos renovaron sus
marcas cuando estas fueron lavadas, y el sitio de marcaje elegido fue su
interfase con otras nutrias y nuevos objetos. Esto sugiere que el
marcaje tiene significado territorial y social desde temprano en la vida
de las nutrias machos. Las hembras, sin embargo, parecen defecar en el
curso de sus períodos de actividad, lo que puede ser un precursor del
marcaje temporal de recursos en uso como ha sido observado en Shetland. |
| [Copyright © 2006 - 2050 IUCN/SSC OSG] | [Home] | [Contact Us] |