|
Last Update:
Thursday November 22, 2018
|
| [Home] |
INTRODUCTION Worldwide many zoos and otter centres house Eurasian otters (Lutra lutra). In the wilderness, territorial expansion has been observed throughout Europe and many reports on the distribution or the status of this species are published every year. Less information is available on veterinary care in captive otters. The Otter Breeding Centre of Hunawihr was created in 1991. The original seven otters came from zoos and parks across Europe: Krefeld Zoo (Germany), Norfolk Park (Great Britain), Zurich Zoo (Switzerland) and Torbiera Park (Italy). Since 1991, 49 pups were born in 25 litters with a mean litter size of 1.96. In 16 years, we cared for 59 otters, 4 pups and 24 adult otters died in the Centre, two escaped and six were released. The youngest died at the age of 22 days whereas the oldest died at the age of 14 years and 9 months. 21 adult otters were autopsied. INFECTIOUS DISEASES 1. Bacterial Infections Infections caused 62.5% (15 otters) of all deaths (24 otters) making them the most common cause of death in Hunawihr. Most infections resulted from injuries of enclosure structures or bite wounds from other otters (Fig. 1a, b). Bites generally occurred on the face, the feet, the tail and the genital area. Fights between otters can be extremely violent and can cause severe injuries. Although injuries are often not immediately apparent, they often lead to a systemic infection (septicaemia). As infections which are not treated may result in death, animal keepers should pay special attention to any fight or wound and start a treatment with antibiotics as soon as possible (Tab. 1). Drugs can be administrated orally with food. The use of palatable tablets once daily makes treatmentseasier. In Hunawihr, we can also easily give long acting injectable antibiotics to otters that do not eat. Otters are used to rest and sleep in artificial wooden holts in each enclosure. The holts can be locked and removed. The trapped otter is simply strongly squeezed with a wire net down the holt and injections can be given. An antiseptic spray may sometimes be applied to wounds of cooperative otters.
Our experience with treatment of infections showed, that every time antibiotics were administered within 24h after visible injuries occurred, the otters recovered. Most mild injuries certainly healed unnoticed. But all severe injuries detected too late (purulent or necrotic wounds) did not heal properly and usually led to septicaemia with lethal complications. Thus several otters died from abscesses, fibrinopurulent pleuritis,pleuropneumonia, hepatitis, peritonitis and pyelonephritis (Tab.2). The two otters that died from pyelonephritis presented numerous and voluminous uroliths in both kidneys (see below). One otter died from peritonitis due to a leak from the intraperitoneal transmitter implanted 4 years before (Fig. 2).
Pasteurella multocida was isolated from two otters that died from pleuropneumonia and fibrinopurulent pleuritis. Pasteurella multocida was also suspected in three other otters that died of a fibrinopurulent pleuritis. Bacteriology showed gram-negative bacteria but they could not be cultured. Infections with Pasteurella species generally often occur due to bite wounds or the intake of contaminated food. This can lead to respiratory infection, septicaemia and endocarditis with a very high mortality rate. Salmonella species were isolated from an otter that died from hepatitis. Faecal analyses were carried out in all otters but Salmonella spp were only found in a few of them (S. hadar, S. virchow or S. indiana). All otters were treated with antibiotics (Colistin) in accordance with the pattern of sensitivity. Foot baths were installed and strict hygienic rules established. The faeces were thereafter regularly analysed until they all proved negative. Treatments and hygienic strategies turned out to be very effective as no other otter died or suffered from salmonellosis. The bacteria could have been transmitted from contaminated food. The otters in the Centre are fed with fish and poultry. Raw poultry is a frequent source of Salmonella.
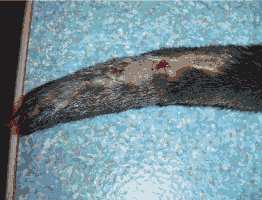
2. Viral Infections Viral infections have never been diagnosed in otters from Hunawihr, no animal have never been tested for viruses. In the early 1990’s some otters were vaccinated against distemper and rabies but vaccinations were stopped, estimating it was not really necessary in captivity. Dogs are not allowed in the Centre since housing of otters started. Canine distemper virus (morbillivirus) is pathogenic for many mustelid species but only very few cases have been described in otters. The status of terrestrial rabies in France is considered to be free since 2001 and vaccination is no longer obligatory (Beuret, pers. comm.). Finally, the immune responses of otters towards the vaccines for dogs have not been evaluated yet. These are the main reasons why the routine vaccination of otters stopped. TRAUMAS Otters often hurt themselves in the enclosures. Regular traumas come from running after one another, fighting, mating, and climbing rocks or trees and escape attempts. They usually show haematomas, contusions or lameness. In these cases anti-inflammatory drugs are administrated orally over three to five days (Tab. 3). Antibiotics are also given if necessary. The otters recover generally quickly, but sometimes traumas can be much more severe. A young female was suffering for at least 24 hours from a traumatic amputation of the tail (Fig. 3). We suspected a sharp-edged steel sheet in the enclosure. She was in a critical state and presented extensive bleeding. We tried to immobilize her with a low dose of anaesthesia (ketamine hydrochloride 5mg/kg) to first treat the haemorrhage and the shock before attempting an operation. Unfortunately she died a few minutes later. The cause of death was most likely a hypovolaemic shock.
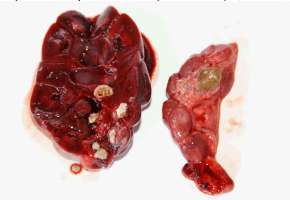
UROLITHIASIS The prevalence of urolithiasis seems high in captive otters. Uroliths were found in the urinary tract or in the kidneys in 61.9% (13 otters) of the autopsied animals (21 otters) in Hunawihr. Most of the time only one kidney was affected; three otters had calculi in both kidneys whereas two other otters had smaller concrements or sand in the bladder and the ureters causing no tract obstructions. Proteus species were isolated from the latter. Renal calculi were generally voluminous and led to atrophy and fibrosis of the renal tissue (Fig. 4). Seven calculi were analyzed using infrared spectrophotometry and all of them were composed of ammonium urate. Bilateral urolithiasis could be found in two otters, together with pyelonephritis and septicaemia.
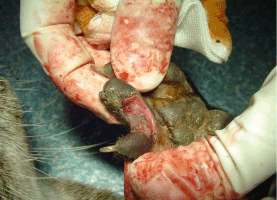
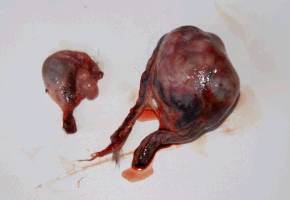
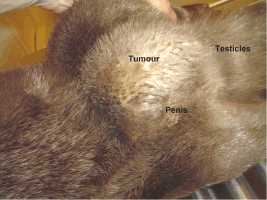
An interesting case was a female otter which drowned a few hours after presenting posterior paralysis. The necropsy revealed nothing but unilateral renal calculi which could have caused great pain, paraplegia and consecutive death. Several other otters have shown sudden posterior paralysis. They have recovered spontaneously. We treated them preventively with acidifying drugs such as ethylene diamine dichlorydrate (300mg/24h) over several weeks. The causes for ammonium urate calculi formation in otters are quite complicated and complex and still not analyzed in all details yet (Weber et al.,1998). MALE GENITAL SYSTEM AFFECTIONS 1. Neoplastic Diseases Two male otters were suffering from genital tumours. They were both older animals (8.5 and 12.5-year-old), cachectic and anorectic. Both were euthanized and autopsied. One had a testis tumour and the other one had a voluminous tumour outside the inguinal canal including the vas deferens with global tissue adhesion in the area (Figs. 5a, b). The testis tumour‘s histological result revealed a Sertoli-cell tumour. The other tumour was not investigated histologically. The testis tumour had been noticed several months before. It was probably not the cause of the health problems from this animal. Sertoli-cell tumours are generally not aggressive. On the contrary, the health of the other otter (8.5 years old) certainly was influenced by the tumour.Its course of illness took only a few weeks and the otter showed much abdominal pain in the end.
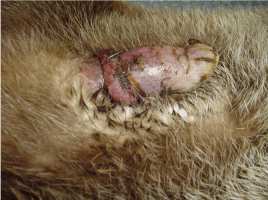
2. Paraphimosis Paraphimosis is an affliction where the prepuce no longer covers the penis. In our case, the problem occured probably due to a bite by the female which shared the enclosure of the 7-year-old male. The pair was observed mating repeatedly for several days. The pain must have caused permanent erection which resulted in the necrosis of the glans penis (Fig. 6). In order to avoid additional complications such as gangrene for example, the otter was captured and treated immediately. The animal was anesthetized (ketamine hydrochloride (10mg/kg) and xylazine (1mg/kg)) and the necrotic parts of tissue were delicately removed. The paraphimosis was reduced by gentle manipulation. Intravenous injections with marbofloxacin (5mg/kg), butorphanol (0.2mg/kg) and meloxicam (0.2mg/kg) were given. Considering his age, and in order to prevent any deleterious consequences of erection or potential urogenital tumours, we castrated him as well. A long acting antibiotic subcutaneous injection with cefovecin (8mg/kg) was given and he was transported to a small enclosure located on the other side of the Breeding Center, away from the females’ enclosures in order to avoid any sexual excitement. Two days after the surgery, the animal showed obvious fever and pain, and the scrotum was oedematous. Anti-inflammatory (prednisolon 1mg/kg) and diuretic drugs (furosemid 8mg/kg) were administrated orally over five days. Three weeks later the otter had completely recovered.
3. Masturbation Three male otters regularly masturbated with both front paws and their mouths. While masturbating they either leaned on their back or their side (Mercier, pers. comm.). Masturbation is often observed among animals, both male and female. It is considered to be a normal behaviour. In our case, boredom or separation from females may have exacerbated sexual stimulation in these captive animals. HEALTH PROBLEMS ARISING DURING BREEDING TIME 1. Deficient Mothers Mothers never had trouble in giving birth but some of them rejected their pups or suffered from agalactia (Capber, 2006). A female rejected her pups despite their incessant cries. Such behaviour is also observed in other carnivores but we do not know precisely why and many hypotheses are possible. Another female showed agalactia in three mammary glands. Only one mammary gland was functional. She could only feed and raise one pup; the others had to be hand-reared. 2. Mortality in Pups Stillbirths and neonatal mortality occurred in 11 pups (22.4%). Four other pups died between the age of 22 and 43 days. One died of starvation because its mother suffered from agalactia (see above) which we did not noticed in time. Another one died of exposure because its mother carried it into the water too early. Finally, two pups were found dead having been crushed by their mothers. 3. Infertility One female is thought to be infertile. We put her together with all possible partners over a period of 5 years but she never got pregnant although matings and copulations were very often observed. There are many causes of female infertility (genetic, anatomic, hormonal, disease, etc.). OTHER HEALTH PROBLEMS 1. Parasites Anthelmintic drugs are administrated prophylactic every spring and fall to all otters (Tab. 4). In numerous faeces analyses from otters in captivity, helminths or eggs of helminths have never been found. However, helminths have been recorded in many spraints of released otters during the post-release surveys. Most of them were nematodes. One cestode and one acanthocephalo specimen was also found, but no further species identifications were realised (Mercier, 2005). No infestation with ticks or fleas has ever been seen in captive otters from the Centre. However, ticks were found on a wild otter captured by hunters near Hunawihr (Manson, pers. comm.). 2. Organochlorine Pesticides and Fipronil Blood samples of two young male otters (2 and 2.5-year-old) anaesthetized for microchip implantation were analyzed for organochlorine pesticides (Tab. 5 ). PCBs (polychlorinated biphenyls) could not be detected in the samples. However, traces of other contaminants like fipronil (agricultural insecticide) were found in very low concentrations. These negative results are not a surprise because captive otters fed a less contaminated diet than wild ones. Otters in Hunawihr are fed with fish (herrings, eels, trouts), chicken (hearts, chicks) and minced meat. Mason (1993) came to the same conclusion after the analysis of contaminants in the spraints of two female captive otters and a pup. 3. Aerophagia A 5-year-old male otter developed chronic aerophagia (Fig. 7) after we moved him to a smaller enclosure. He was presented with significant abdominal bloating, belching and flatulence after he suddenly started to swallow air over up to ten minutes while swimming in circles rapidly. This would happen several times each day and most of the time he recovered spontaneously. Aerophagia did not seem to bother him at all; he just faced difficulty swimming underwater. We treated him with activated charcoal when the abdomen was extremely swollen. First we thought that the cause could have been psychogenic. So we tried anxiolytic treatment using clomipramine (1.5mg/kg/24h) and replaced him in a larger enclosure with a female. The treatment did not prove effective. His state improved temporarily after we gave him daily supplements with lactic fermentative bacteria (Lactobacillus acidophilus). These symptoms were not further investigated; the animal is still presenting aerophagia from time to time but seems very well otherwise.
4. Old Age In captivity, otter can live very old. A female otter died at the age of 11 years and 6 months whereas a male otter died at the age of 14 years and 9 months. They presented no particular health problems except for age-related sclerosis of the crystalline lens observed in all old otters. The necropsies failed to show any particular cause of death. 5. Unknown Causes of Health Problems or Deaths Other minor health problems such as sporadic vomiting, diarrhoea, anorexia, rhinitis, conjunctivitis and alopecia were managed using symptomatic treatments. The causes of these problems were unknown and the animals generally recovered rapidly. 12.5% (3 otters) of all deaths could not be related to precise causes. Either the animals were not autopsied (1 otter) or the necropsies and bacterial cultures failed to show any cause of death (2 otters). ANAESTHESIA Otters are generally anaesthetized for two purposes: surgeries and subcutaneous microchip or intra-peritoneal transmitter implantations. The otters are trapped in an artificial wooden holt (Fig. 8) and strongly squeezed with a wire net down the box in order to be immobilized. They are anaesthetized with an intramuscular injection in the thigh with ketamine hydrochloride (10mg/kg) and xylazine (1mg/kg) or acepromazine (0.25mg/kg). If a surgery is undertaken, a tube is inserted in the trachea and anaesthesia is prolonged using isoflurane (2-4%). Breath and heart frequencies as well as rectal temperature are monitored. Antibiotic and anti-inflammatory drugs are injected intravenously. If the surgery turns out to be painful we also use opiate analgesic such as butorphanol (0.2mg/kg). This procedure works well, only one otter died under anaesthesia (out of 12 procedures). Death occurred because of the poor condition of the animal before anaesthesia. One otter in good condition died several hours after anaestensia with no obvious reason. After anaesthesia the otters are put back into the holt and kept warm until they are fully awake. They are generally transported to their enclosures at the same day.
DISCUSSION This paper presents an overview about diseases and health problems we had to deal with in an otter breeding centre. It is interesting to point out that much of the diseases are also reported in wild otters whereas specific problems arise from captivity. Bite wounds are also common in wild otters (Simpson, 2006, 2007; Green and Green, 2002). Eurasian otters are solitary and competition for territory often results in violent intraspecific aggression between male otters. Some heavy infections may even lead to septicaemia and death. In captivity, otters sharing the same enclosure are females, a pair or a pair with pups. In Hunawihr, every time we tried to put two males together they became aggressive and had to be separated again. Therefore most aggressions occur between female otters. Females with pups were seen trying to bite the male when getting too close towards the pups. Bites can also happen in otters of both sexes during mating. However, in the wild, road traffic accidents have been identified to be the major cause of death (Lanszki, 2007; Simpson, 2006; Green and Green, 2002; Madsen et al.,1999) whereas the most common cause of death in captivity is a bacterial infection. We isolated Pasteurella multocida from the lung of an otter that died from pleuropneumonia. Specific infectious diseases are rare in both captive and wild otters. In Hunawihr we had to cope once with Salmonellosis. Borg (1964) described human tuberculosis in a captive otter. Distemper virus was found in two captive otters (Geisel, 1979). Loupal et al. (1998) found two wild otters that died from canine distemper and Madsen et al. (1999) recorded distemper virus in six wild dead otters but they seemed not to have suffered from the disease. Mañas (2007) revealed the possible role of wild otters in the dissemination of Aleutian disease on other riparian species. Fournier et al. (2004) found antibodies to ADV (Aleutian disease parvovirus) in many wild mustelid species from south-western France, but no otter is recorded. Rabies and toxoplasmosis may also threaten wild otter populations (Jacques, 2007). Park et al. (2007) described for the first time a canine adenovirus type 1 (CAV-1) in a 10-year-old captive otter. Ammonium urate urolithiasis is commonly found in captive and wild otters (Madsen et al., 1999; Weber et al.,1998). It may be due to a specific purine metabolism in otters (Weber et al., 1998). Kidney calculi may cause severe back pain. In our study most of the otters showed calculi in only one kidney and no death could be directly attributed to renal failure in these cases. Urolithiasis is also common in captive Asian small-clawed otters Aonyx cinereous (Petrini et al.,1999; Féjan and Hue pers. comm.), but the main component in this species is calcium oxalate. Parasites have often been described in wild otters (Torres et al.,2004; Madsen et al., 1999; Torres et al.,1999; Vismanis and Ozolins, 1998; Fons and Feliu, 1996). Matsuda et al. (2003) found Dirofilaria immitis in the heart of a young otter and suspected the otter to be a definitive host for this worm. Simpson et al. (2005) found flukes (Pseudamphistomum truncatum) in the gall bladders of a few otters. As we mentioned, ectoparasites are uncommon in captive otters as well as in wild ones (Madsen et al.,1999; Green and Green, 2002). However, we did find ticks on a wild otter captured near Hunawihr. Neoplastic diseases were not often described in Eurasian otters. Weber and Mecklenburg (2000) reported a malignant melanoma in a 11-year-old female otter and Bae et al. (2007) reported a hepatocellular adenoma in a 7-year-old captive female otter. The two otters that developed neoplastic diseases in Hunawihr were both over 8 years old. Life expectancy in captive otters is two to three times longer than in wild ones. Wild otters usually do not live more than 5 years whereas captive animals can live up to 19 years (Sidorovich, 1991; Pechlaner and Thaler, 1983). As prevalence of neoplastic diseases is higher in older animal and human populations, tumours must certainly occur more frequent in captivity. Finally ocular problems such as blindness and retinal dysplasia were also described in Eurasian otters (Williams et al.,2004; Williams, 1988) as well as neurological problems such as hydrocephalus and meningitis (Green and Green, 2002). CONCLUSION Veterinary care of captive otter is challenging. Dealing with unhabituated animals that cannot be handled for diagnosis and treatments is not easy. The lack of medical information about the species and the necessary control of the zoological facilities’ spending are additional obstacles for veterinarians. We certainly do need more investigations on many diseases and health problems. The haematology and biochemical reference intervals determination for the Eurasian otter by Moran et al. (2001) is a good example of what can be done to improve our knowledge about this discipline for instance. Information gained from captive otters may be of importance for all otters and for all veterinarians dealing with these animals. ACKNOWLEDGMENTS - Many thanks to Laurent MERCIER from the Centre de Reproduction des Loutres d‘Hunawihr, DVM Hélène JACQUES from the IUCN Otter Specialist Group and DVM François MOUTOU from the Agence Française de Sécurité Sanitaire des Aliments (AFSSA) for reading the draft of this paper and giving me advices. Thanks to Alexandre LEHMANN and Wandrille BEXON from the Centre de Reproduction des Loutres d‘Hunawihr for their help in gathering documentation. Thanks to DVM Christine MANSON from the Laboratoire Vétérinaire Départemental (LVD), DVM Sylvie LAIDEBEURE from the Parc Zoologique de Paris (MNHN), Charles LEMARCHAND from the Laboratoire de Biologie des Prostistes (CNRS) respectively for the autopsies and bacterial analyses, some calculi determination and the analysis of blood contaminants. Thanks also to DVM Alexandra Outlaw for the English correction. REFERENCES Bae, I.-H., Pakhrin, B., Jee, H., Shin, N.-S., Kim, D.-Y. (2007). Hepatocellular adenoma in an Eurasian otter (Lutra lutra). J. Vet. Sci. 8(1): 103-105. Résumé : Resumen: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [Copyright © 2006 - 2050 IUCN/SSC OSG] | [Home] | [Contact Us] |