|
Last Update:
Thursday November 22, 2018
|
| [Home] |
Received 12th January 2007, accepted 28th August 2007
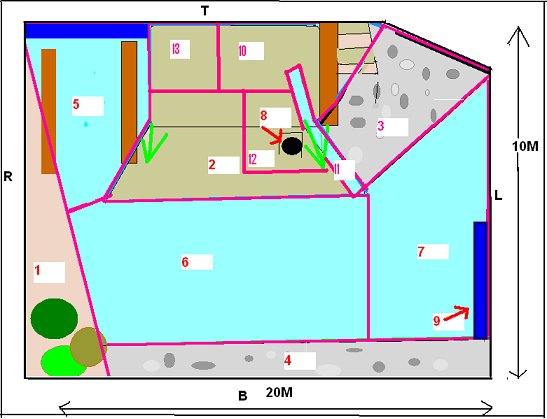
INTRODUCTION Research Question The study zoo had noticed that their Asian otters constantly displayed ‘begging’ behaviour towards staff and members of the public, even during and immediately after feeding. This was felt to be a welfare issue and the zoo staff was interested in elucidating the cause behind this behaviour. Previous research on begging in Asian short-clawed otters Asian otters have been observed ‘begging’ in a variety of captive environments (Maslanka and Crissey, 2002). However despite the widespread occurrence of this problem, there has been little published research into the underlying cause. In 1982 Markowitz and Foster-Turley used environmental enrichment in the form of live prey to reduce abnormal behaviour in Asian otters. They suggested that otters ‘begged’ because they were bored and the behaviour induced a response from visitors. Steen et al. (1995) reduced ‘stereotypical begging’ in Asian otters by randomly distributing food in time and place with the use of a catapult. This reduced dependence on keepers for food and added an element of unpredictability and control over their environment. Owen (2004) observed that the otters ‘begged’ from visitors when they were hungry and that visitors threw food into the enclosure reinforcing the behaviour. However this was an ad libitum observation as part of another study and the statement was not statistically tested. Although the previous research was successful at reducing the ‘begging’ through environmental enrichment, none of the previous studies looked into elucidating the cause at the root of the behaviour. Therefore the purpose of this research was to determine the proximate cause of ‘begging’ behaviour in a group of captive Asian short-clawed otters. Hypotheses H1: Boredom is the proximate cause of ‘begging’ behaviour as a result of lack of stimulation and opportunity to engage in the appetitive component of feeding behaviour. H2: Hunger is the proximate cause of ‘begging’ behaviour as a result of inadequate nutrition and a feeding regime that doesn’t take into account the natural foraging ecology of Asian otters. Boredom induced ‘begging’ Inglis et al. (1997) found that animals prefer to work for food in a phenomenon called ‘contrafreeloading’. It is possible to meet an animal’s entire physiological requirement and yet often they often will develop abnormal behaviour because of stress and boredom (Poole, 1992). A study into the behaviour of young rhesus macaques found that they exhibited less self-directed behaviour and were more exploratory when they were allowed to work for food (Chamove, 1989). In nature, working for food provides information about resource availability and through evolution it has become a rewarding activity in itself (Poole, 1992). Mammals rely for their survival on collecting and analyzing data and acting intelligently. Their psychological well-being depends upon having an environment that offers facilities to search for information to establish and monitor their concept of the world. Opportunists suffer more in captivity because they are adapted to highly variable environments and captivity does not provide enough stimulation. Social animals have higher cognitive abilities, which, also increases the need for constant sources of stimulation (Mench, 1998; Robinson, 1998). The Asian short-clawed otter is an opportunist and highly sociable and therefore it is possible that the captive environment is not stimulating enough for them (Kruuk et al., 1994). Behavioural problems associated with feeding may develop because foraging constitutes the main form of information gathering for otters, which spend 41 - 60% of their time in the wild involved in feeding or foraging activities (Davis et al., 1992; Spelman et al., 1999; Kruuk 1995, Hoover and Tyler, 1986). Hughes and Duncan (1988) and Jensen and Toates (1993) argue that animals will suffer if they are unable to perform behaviour that they are motivated to do, even if it is not necessary to meet their immediate physiological requirements. For any behaviour that is largely governed by internal factors, motivation levels will sooner or later increase above threshold. This will trigger appetitive behaviour but in some environments it will be impossible to reach the consummatory phase so the appetitive behaviour will continue, sometimes in an abbreviated form. It can result in boredom, redirected behaviours, vacuum activities, stereotypes and reduced health (Veasey, 1996). Carnivores devote a large amount of time and energy to hunting behaviour in the wild. In captivity there is little opportunity to express hunting behaviour while a strong motivation remains (Lyons et al., 1997). Shephardson et al (1993) found that providing small felids with hidden food satisfied the need to express foraging behaviour as well as information gathering. If the lack of stimulation and opportunity to engage in the appetitive phase of feeding behaviour hypothesis is correct, stimulating the otters hunting/foraging behaviour with live prey should reduce begging because it provides them with an outlet for foraging motivation and a more stimulating environment for information gathering thus negating the motivation to seek stimulation from outside the enclosure and allowing the appetitive component of feeding behaviour to progress to the consummatory phase. Hunger induced ‘begging’ The second hypothesis suggests that poor nutrition or a feeding regime that does not take into account the natural foraging ecology and the physical adaptation of the species could result in hunger. It has been well documented that animals fed restricted amounts of food will develop behavioural stereotypes (Lyons et al., 1997). The stereotypes are built upon elements of redirected activities consecutive to thwarted attempts to reach food and may have been an expression of foraging motivation (Terlouw et al., 1993). Broiler hens have been found to excessively drink, preen and peck non food objects when food was restricted (Savoury et al., 1992). Pregnant sows living in food restricted environments developed pre-feeding stereotypes (Terlouw et al., 1993). Wild Otters eat roughly 20% of their body weight per day (Duplaix-Hall, 1975). However the study group ate 384g per day each, which is only 12.5% of their estimated body weight. This is 36% less than they would eat per day in the wild. The dominant female was also lactating during the study period but no extra food was provided to minimize the weight loss and metabolic stress associated with milk production, despite recommendations to do so by Tumarov and Sorina (1997). Asian otters have high metabolic rates (Borgwardt and Culik, 1999). Food is digested and defecated within one hour of ingestion and they eat frequently throughout the day (Lekagul and McNeely, 1988). Therefore Lombardi (2002) recommends that Asian otters are fed three times a day or more due to their natural feeding style of frequent small amounts, fast metabolism and generally high activity levels. The study group were fed roughly four times per day, however the difference between zoo feeding regimes and the natural foraging ecology and morphological adaptations of the Asian otter may mean that even four feeds per day are not frequent enough to prevent ‘begging’. If the hunger hypothesis is correct then increasing the quantity of food to 20% of the body weight and feeding more frequently throughout the day will reduce hunger and negate the need to ‘beg’ because it more naturally represents their foraging ecology and morphology. METHOD Study area The outside enclosure is approximately 20m x 10m (Figure 1). There is an underground burrow, where the otters can not be seen by the public (area 8) and have open access to throughout the day. There are two sandy areas (1) and (2,10,12,13) and 2 pebbled banks (3 and 4). A waterfall runs from the top of the enclosure into the pool (11). These areas are separated by a large pool of water that flows through the enclosure (5, 6 and 7). There is also a bridge which they can go underneath (9). The otters can be viewed by the public from all sides although the viewing areas are set approximately 1m back on three sides, with only close proximity at the top end.
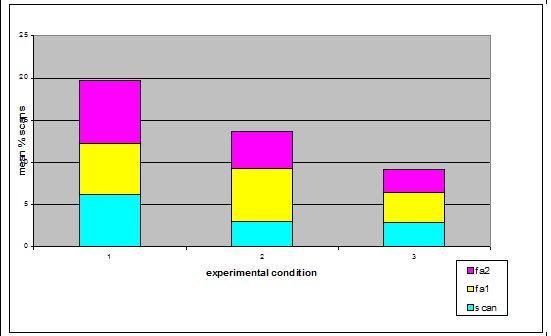
Study subjects There were three adult Asian short-claws, five juveniles and five pups. The pups were five weeks old when the study began and therefore were not included in the study because they spent most of their time in the burrow and were not independent of their mother Mia, who was lactating during the study. The eight remaining otters were observed. See Table 1) ‘Begging’ definitions Steen et al. (1995) referred to begging as a stereotypical movement. However the ‘begging’ observed in this study group was not an invariant functionless behaviour and it was not strictly repetitive. Therefore for the purpose of clarification, in this study the broad term ‘begging’ has been replaced with the term ‘feeding anticipation behaviour’, which has then been graded on 3 levels: scan, FA1 and FA2 (refer to Table 2 for operational definitions).Research design The method is based on a study by Repp et al. (1988) in which stereotypic and self injurious behaviour in humans were treated based on hypotheses about their cause. A baseline was used to formulate hypotheses and two groups were treated based on alternative hypotheses. The cause could then be determined by the response of the groups to treatment. For the purposes of this study both treatments were applied to the same group of otters due to lack of availability of two comparable groups. To reduce the confounding effect of applying one treatment after another, two days were left between treatments to allow behaviour to return to baseline. The experiment was split into three experimental conditions. In Condition 1 the normal husbandry regime was observed to provide baseline data. In Conditions 2 and 3 the feeding regime was manipulated based on two alternative hypotheses; Details of experimental conditions in Table 3. Lombardi (2002) argues that it is important to look at how other zoos manage the husbandry. Therefore questionnaires were sent out to zoos housing Asian otters requesting information regarding feeding regimeData collection and analysis Group scans were conducted every fifteen minutes on the instant between 8am and 5pm. For each experimental condition 180 scans were taken over 45 hours of observations. The ID, nearest neighbour, nearest neighbour distance, body orientation, behaviour and vocalisation were noted down for every otter. Visitor numbers and weather conditions were also taken in every scan, as well as any other relevant details such as keeper presence or disturbances. SPSS was used to analyze the data; non-parametric statistics were used because of the relatively small sample size and because the data were not normally distributed. Friedman and Wilcoxon matched pairs tests were used to determine significant differences between conditions for the group because the data was related. Appropriate graphs were then produced to demonstrate significant trends using SPSS and Excel (Hawkins, 2005). A critical significance level of P=0.05 was used. RESULTS Activity Budgets The group data was collated for each stage and the mean percentage of group scans between 8am and 5pm calculated for each behaviour for the group. The results indicate that there were significant differences in time budget between experimental conditions. The percentage of group scans in which any feeding anticipation was recorded declined significantly from 21% in Condition 1 (baseline) to 14% in Condition 2 (Wilcoxon, Z=2.366, N=8, P=0.016) (Figure 2). Feeding anticipation declined further to 9% in Condition 3, this was significantly less than that in both Conditions 1 and 2 (FA: con 1 and 3, Wilcoxon, Z=2.383, N=8, P=0.017) (FA: con 2 and 3, Wilcoxon, Z=2.383, N=8, P=0.035). The mean percentage of group scans in which foraging behaviour was recorded increased significantly from 9% in Condition 1 to 24% in Condition 2 (Foraging Con1 and 2: Wilcoxon Z=2.524, N=8, P=0.012) (Figure 2). Foraging behaviour then decreased to 12% in Condition 3, which was significantly less than in Condition 2 (Foraging Con2 &3: Wilcoxon, Z=2.521, N=8, P=0.012). However there was significantly more foraging in Condition 3 than in Condition 1(Foraging Con1 and 3: Wilcoxon, Z=2.521, N =8, P=0.012). Effect of experimental condition on feeding anticipation behaviour
The mean percentage of group scans in which the group was displaying
scanning behaviour was significantly less in both Conditions 2 and
3 compared to Condition 1 (Scanning Con 1 and 2: Wilcoxon, Z=2.383,
N=8, P=0.03)( Scanning Con 1 and 3: Wilcoxon, Z=2.243, N=8, P=0.025)
(Figure 3). There was not a significant difference between Conditions
2 and 3 (Scanning Con2 and 3: Wilcoxon, Z=0.690, N=8, P=0.490).
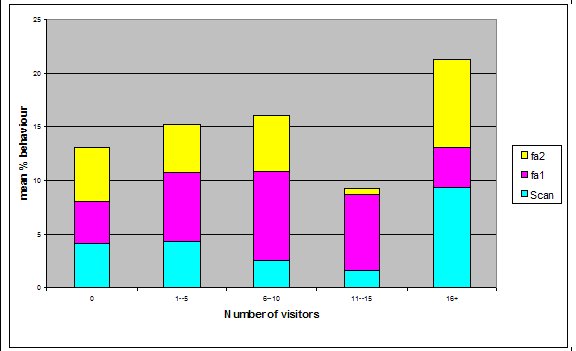
Effect of visitor density on feeding anticipation Feeding anticipation behaviour differed slightly between different numbers of visitors, the highest density visitor group does have the highest feeding anticipation, and however the second largest group had the lowest mean % scans in which the otters were displaying feeding anticipation (Figure 4). There was no significant difference between visitor numbers and the mean % scans in which feeding anticipation was present (Friedman: χ2=5.438, df=4, P=0.245).
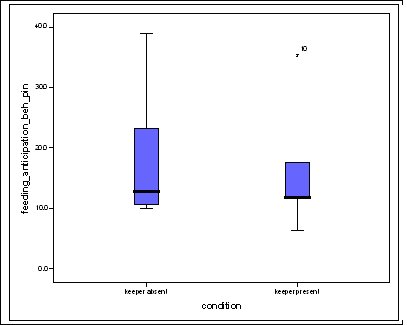
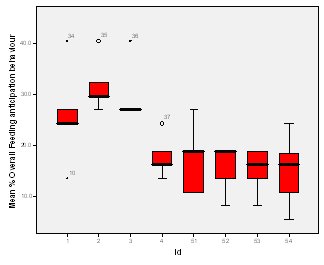
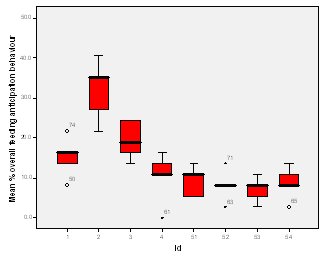
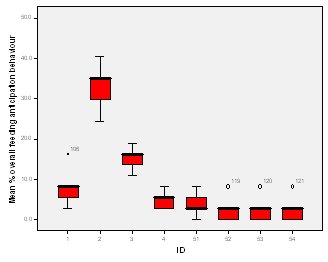
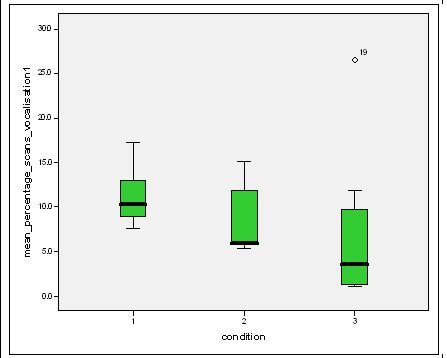
Feeding anticipation behaviour was slightly greater when no keepers were in sight (Figure 5). However this was not statistically significant (Wilcoxon signed ranks, Z=-0.140, N=8, P=0.889). Differences in feeding anticipation behaviour within the group Feeding anticipation changed with experimental condition for each individual and highlight differences within the group (Fig. 6-8). There is variation within the group in Condition 1 (Figure 6). The adults (1-3) show significantly greater feeding anticipation behaviour than the juveniles (4-54) (FA Con1 Adults–Juveniles: Mann-Whitney, U=0.000, n 1:5 n 2:5, P=0.009). Mia (2) showed the greatest feeding anticipation behaviour. However there was not a significant difference within the adults or within the juveniles (FA Con1 ID 1-3: Kruskal-Wallis, χ2 = 3.933, df = 2, P=0.140) (FA Con1, ID 4 -54: Kruskal- Wallis, χ2 = 0.962, df = 4, P=0.916). All individuals were anticipating food less in Condition 2 than Condition 1 (Figure 7) except for Mia (2) who still exhibited high feeding anticipation behaviour, which was significantly higher than the other adults (1 and 3) (Con2: ID: 2and 3:. Kruskall Wallis, χ2=4.870, df = 1, P=0.027) (Con2: ID: 1 and 2: Kruskal-Wallis, χ2=6.400, df=1, P=0.011). There was no significant difference between the two adult males (Con2: ID 1 and 3: Mann-Whitney U, U=6.500, n 1: 5 n 2: 5, P=0.202). The adults (1-3) showed significantly greater feeding anticipation behaviour than the juveniles (4-54) (Con2: adults – juv: Mann- Whitney U, U=0.000, n1.5 n1.5, P=0.009) There was no significant difference between juveniles (Con2: ID: 4 – 54: Kruskal-Wallis, χ2=2.609, df=4, P=0.625). Feeding anticipation behaviour was further reduced in condition 3 from conditions 1 and 2 for all individuals except Mia (2) (Figure 8). The juveniles showed the greatest overall reduction in feeding anticipation behaviour. The adults showed significantly greater feeding anticipation than the Juveniles (Con3: ID: adults and juv:. Mann- Whitney U, U= 0.000, n 1: 5 n 2: 5, p=0.009). There was a significant difference in feeding anticipation behaviour between all the adults in Condition 3 with Mia (2) demonstrating the greatest feeding anticipation followed by Ollie (3) and Mike (1) (Con3: ID: 1 and 2: Mann-Whitney U, U=0.0005, n 1: 5 n 2: 5, P=0.008) (Con3: ID: 1 and 3: Mann- Whitney U, U=3.000, n 1: 5 n 2: 5. P=0.04) (Con3: ID: 2 and 3: Mann- Whitney U, U=0.000, n 1: 5 n 2: 5, P=0.008). There was no significant difference between the juveniles (Con3: ID: 4 – 54: Kruskal- Wallis, χ2=2.935, df=4, P=0.56). Vocalisations Effect of experimental condition on vocalisation 1 The mean percentage of group scans in which the group were vocalising at intensity 1 shows that vocalisation 1 decreased in Condition 2, and further in Condition 3. There was a significant difference between Condition 1 and 2 but not between Condition 1 and 3 and Conditions 2 and 3 (V1: Con 1 and 2: Wilcoxon, Z=2.383, N=8, P=0.017) (V1: Con 1 and 3: Wilcoxon, Z=1.183, N = 8, P= 0.237) (V1: Con 2 and 3: Wilcoxon, Z=1.402, N=8, P=0.161).
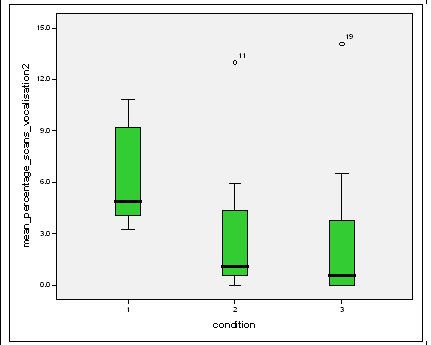
Effect of experimental condition on vocalisation 2 The mean percentage of group scans in which vocalisation 2 was recorded decreased in Conditions 2 and 3 from Condition 1. There was a significant difference between Condition 1 and 2 and Condition 1 and 3 but not between Conditions 2 and 3 (V2: Con 1 and 2: Wilcoxon, Z=2.383, N=8, P=0.017) (V2: Con 1 and 3: Wilcoxon, Z=2.243, N=8, P=0.025) (V2: Con 2 and 3: Wilcoxon, Z=0.690, N=8, P=0.490).
Zoo Questionnaire Out of seven zoos the quantity and type of food provided was similar across all institutions, although ‘begging’ was observed to some extent in all of them (Table 5). The nutritional content of the 3 main foods eaten by the study otters was adequate according to guidelines by Lombardi (2002) (Table 6).
Visitor density Visitor density and intensity have been found to affect the behaviour of zoo animals (Foster-Turley, 1982; Marguilis et al., 2003; Sellinger; 2005; Hosey and Druck, 1987; Chamove et al, 1988). Therefore the number of visitors was recorded during every scan to measure the effect of this possible confounding variable. The results showed that there was no significant difference in feeding anticipation between visitor densities ranging from 0 to 16+ (Refer to Figure 4). This suggests that visitors had no effect on feeding anticipation behaviour therefore they were not a confounding variable. From ad libitum observations I noticed that the otters largely ignored the visitors, often trying to see past them to the path where food arrived from. The only visitors that they ‘begged’ directly from were wearing blue shirts in the same shade as that worn by the keepers. This contradicts findings by Owen (2004) who found that Asian otters only ‘begged’ in the presence of people. Research into the intensity of visitor interactions on Asian otter ‘begging’ behaviour may be an avenue of further study.Keeper presence It was felt that keeper presence may confound the results of the study. Therefore when the keepers were present during a group scan it was recorded along with the nature of their visit. The results showed that there was no significant difference in feeding anticipation behaviour between scans when the keeper was present and absent (Figure 5). This result was initially surprising as it appeared that the otters would nearly always ‘beg’ from keepers. The result may have been because the majority of instants where the keepers were present, they were carrying out maintenance on the enclosure and the otters were more curious as to what the keeper was doing rather than begging for food. They may have also picked up on the cue that when the keeper is in the enclosure they are not going to be fed, as they were always fed from the outside, therefore they could relax knowing that they would not be fed. This provides tentative support for the proposal by Waitt et al. (2001) that reliable cues as to when feeding will and will not occur allows animals to relax and engage in non anticipatory activities.Difference in feeding anticipation behaviour within the group The results of differences within the group showed that in every condition the adults showed significantly higher levels of feeding anticipation behaviour than the juveniles. This lends support to hypothesis one (lack of stimulation). Abnormal behaviour caused by captive environment is often more difficult to reduce in adults even when the environment becomes enriched because the behaviour pattern is more fixed (Wemelsfelder, 1993). However Mia (2) consistently ‘begged’ more than all the other otters and showed the least reduction in feeding anticipation behaviour. She was lactating to provide milk for a litter of five during the study and therefore her energy requirements would have been the greatest. Even in Condition 3 when the otters were fed 20% of their body weight based on the amount they eat in the wild, this did not account for Mia’s lactation needs and therefore it is plausible that she was still hungry. The fact that Mia did not improve and ‘begged’ significantly more than the other adults in Conditions 2 and 3 supports hypothesis two (hunger).Vocalisations Vocalisations often accompanied feeding anticipation, particularly vocalisations 1 and 2. Vocalisation 1 was less shrill and lasted for a shorted duration than vocalisation 2 and was more often associated with less intense feeding anticipation behaviours. There was a significant reduction between Condition 1 (baseline) and the two experimental conditions for both vocalisations but not between Conditions 2 and 3. The fact that there is not a significant difference between Conditions 2 and 3 in vocalising did not lend support to either hypothesis over the other. The fact that there was a significant difference between baseline and both experimental conditions suggests that feeding anticipation may be due to both hunger and lack of stimulation.Time budget The group data was collated for each stage to produce graphs on time budgets to help to determine if behavioural diversity increased and if abnormal behaviour and inactive behaviours were reduced (Figure 2). The results show that abnormal behaviour and rest declined significantly and foraging significantly increased in Condition 2 when stimulation in the form of foraging for live insects was introduced. This supports hypothesis one (lack of stimulation and of opportunity to engage in appetitive behaviour; result: ‘begging’ behaviour). However, play behaviour decreased and instances of ‘out of sights’, where the animals were in the burrow, usually resting, increased. This provides evidence in favour of hypothesis two because the increase in out of sights suggests a greater need to rest possibly due to the energetic demands of the extra foraging opportunity. The decline in play behaviour adds extra weight to this argument because play behaviour can be an indicator of nutritional status. For example, studies by Barrett et al. (1992) and Sommer and Mendoza Granados (1995) found that play behaviour was strongly correlated with habitat quality in monkeys. Good habitat quality leads to better nutritional status because the food is more plentiful and nutrient rich. Animals with better nutritional status play more because they can afford to engage in this luxury behaviour.In Condition 3, play behaviour increased significantly from Condition 2 back to baseline levels. Foraging behaviour declined in Condition 3 although it was still significantly greater than in Condition 1. The fact that foraging behaviour did not decline to baseline levels in condition three might have been due to a residual motivating effect from Condition 2 or it could be interpreted as an increase in activity due to more energy from a more appropriate husbandry routine, therefore supporting both hypotheses. However percentage time resting and ‘out of sight’ (behaviours characterized by inactivity) were both significantly lower in Condition 3 than the other two Conditions, which supports hypothesis two (hunger) because they were engaged in more active behaviour as a result of receiving more energy in their diet despite the fact that there was little stimulation in their environment. Feeding time I was unable to analyze the correlation between feeding time and feeding anticipation behaviour because the feeding times were too varied in Conditions 1 and 2 for a particular time of day to be positively correlated with feeding anticipation. This inadvertently led to a possible explanation as to why they ‘begged’ so frequently and for such long periods of time. They were routinely fed four meals a day, however on two out of ten occasions the afternoon feed was recorded as missed. Furthermore on five out of twenty feeds the keeper had presumably fed the otters before observations began in the morning or after they finished in the evening creating the possibility of other missed feeds. The morning feed was recorded to occur at times between 8.00-10.00, the lunchtime feed between 10.00 -12.30, the afternoon 12.30-14.30 and the evening 16.15 -16.45. This meant that there was the possibility that the otters might be fed in 7 out of 9 hours of observation during the day. The schedule might have been too unpredictable for Asian otters to rely on cues informing them when feeding would definitely not occur and therefore they were in a constant state of alert, unable to relax. This theory is supported by Waitt (2001) who argues that animals pick up on external cues to reliably predict feeding time and these cues cause the change in behaviour prior to feeding. The safety-signal hypothesis suggests that reliably signalling to an animal prior to delivering food allows the animal to predict when not to anticipate the event allowing the animal to relax during times that it knows it will not be fed. Unreliable signals and unpredictable feeding times result in the animal anticipating the delivery of the stimuli which may or may not be forthcoming causing frustration. Waitt (2001) concludes that if animals had a clear distinct and reliable signal that informed them about the event, then pre-feeding behaviours might not occur. This is an interesting area for a long-term study; It would be interesting to determine how feeding the otters on a truly predictable schedule with reliable pre-feeding cues over a long period of time would affect their feeding anticipation behaviour. Mia: Adult female Mia the alpha female in the group was lactating during the study and unique in much of her behaviour. She typically ‘begged’ for longer periods and more intensely than the other otters and her play was characterized entirely by pebble rolling. She also performed a type of pebble rolling behaviour that was unique to her in the group. She would pick up pebbles and extend her paw towards the perimeter of the enclosure and then pull it back placing the pebble in her mouth repeating the sequence often for prolonged periods of time. The behaviour looked very analogous to human ‘begging’ and may have been reinforced by the public who thought she was ‘begging’ for food creating an association between the behaviour and acquiring food. However, no visitors were observed feeding the otters during the study. The correlation between feeding time and frequency of pebble rolling could not be analyzed because of the great variation in feeding time. However from ad libitum observations the behaviour increased in intensity the longer the period without food has been. The behaviour may be a form of coping with the stress or an expression of frustration about lack of control over feeding opportunities and therefore a welfare concern. Further study is required to establish the cause of this behaviour and if indeed it is a form of oral stereotypy. Zoo Questionnaire In seven zoos the quantity and type of food provided was similar across all institutions, although begging was observed to some extent in all of them indicating that it’s possible that they all underfeed their otters (Table 5). The nutritional content of the 3 main foods eaten by the study otters was adequate according to guidelines by Lombardi (2002) (Table 6). However there are no formal guidelines on the quantity of food to feed otters, therefore they may not be receiving enough to provide the quantity of nutrients needed, which may in turn lead them to beg for food. Limitations of the study The adult female was lactating during the study which may mean that feeding anticipation levels were higher than in times of less physiological stress. All three conditions were studied on the same group of otters, possibly making the results dependent on each other. However it is very difficult to find groups that are similar in enough respects to do a comparative study without further factors confounding the results. The relatively small sample size of eight otters makes it difficult to generalize about all Asian otters in captive environments, therefore reducing the application of the findings to the study zoo. The study is also limited by its short-term nature. Ideally it would run over several months to determine the long term effect of each condition and prior to the study an investigation into the efficiency of digestion of the different food items fed to the otters would have occurred. It may be that they digest certain food items badly and therefore are hungrier on the days they are fed them. However this was not possible because of time constraint. CONCLUSIONS In conclusion, I get the impression from the results that Hypothesis 2, hunger, is the major proximate cause of ‘begging’ behaviour. However boredom as a result of lack of stimulation and opportunity to perform the appetitive components of feeding was also a major factor. Foraging enrichment could help alleviate ‘begging’ behaviour by providing a distraction and giving the animal a sense of control over the environment. Thus reducing the frustration associated with captive feeding. This will only be effective if hunger levels are not too high. In the case of Mia hunger levels definitely have been too high. The inconsistency in feeding times may have also contributed to the extent of the feeding anticipation behaviour although this needs further study. RECOMMENDATIONS
ACKNOWLEDGMENTS - I would like to thank Lesley Wright for her personal communication and Dawn Hawkins at ARU for helping me with the statistical analyses. REFERENCES Allen, M.E., Oftedal, O.T. (1997). Essential nutrients
in the mammalian diet. In. Kleiman, D.G. (ed) Wild
mammals in captivity: principles and techniques. University of Chicago
press, Chicago. Résumé : Quelle Est La Cause Proximale Du Comportement De Mendicite Observe Chez Un Groupe De Loutres Cendrees Captives ? Resumen: ¿Cual Es La Causa Inmediata Del Comportamiento De Mendigueo En Un Grupo En Cautiverio De Nutrias De Río Asiáticas De Garras Cortas? |
||||||||||||||||||||||||||||||||||||||||||||||
| [Copyright © 2006 - 2050 IUCN/SSC OSG] | [Home] | [Contact Us] |