IUCN/SSC Otter Specialist Group Bulletin

|
IUCN/SCC Otter Specialist Group Bulletin Volume 27 Issue 1 Pages 1 - 57 (January 2010) Citation: Sherrard-Smith, E. and Chadwick, E.A. (2010). Age Structure of the Otter (Lutra lutra) Population in England and Wales, and Problems with Cementum Ageing. IUCN Otter Spec. Group Bull. 27 (1): 42 - 49 Age Structure of the Otter (Lutra lutra) Population in England and Wales, and Problems with Cementum Ageing Eleanor Sherrard-Smith1 and Elizabeth Chadwick1 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff, CF10 3AX, UK. Email: Chadwickea@cardiff.ac.uk |
 |
|
Abstract: Age is an important parameter in understanding population structure and age-dependent processes such as accumulation of contaminants. In the current study, canines and incisors of sub-adult and mature wild otters (Lutra lutra) from England and Wales were sectioned and incremental cementum lines were used as an indication of age. The age structure of the sample population is much younger than some European populations (of 110 otters aged, only 10 were aged four or older). Cementum ageing is useful here in giving a broad indication of age structure, but is imprecise for species which do not exhibit seasonal breeding. Age is likely to be underestimated in most cases. |
| Keywords: Age structure; Cementum analysis; Road-traffic casualties; Otter, Lutra lutra |
| Française | Español |
INTRODUCTION
Many conservation goals require an understanding of population age structure, age-specific reproduction, growth and survival (Bodkin et al., 1997, Harris et al., 1992, Stoneberg and Jonkel, 1966). A range of methods for ageing wild animals are described in the literature e.g. size frequencies (Tanaka, 1956), lens weight (Teska and Pinder, 1986), degree of tooth wear (Brothwell, 1989), and cementum banding (Castanet et al., 1990); here, we discuss the use of cementum banding in ageing Eurasian otters Lutra lutra.
Lieberman (1994) describes the biological basis for seasonal cementum banding. Cementum is a bone-like connective tissue that grows in incremental layers around the root of teeth. Banding patterns are caused by changes in mineralization and collagen orientation; these can be caused by nutritional or biomechanical factors that vary on a seasonal or annual basis. There have been a number of controlled studies indicating that cementum banding can be used to accurately age mammals including buffalo (Moffit, 1998), harbour seals (Norgaard and Larsen, 1991), stoat (King, 1991), and badger (Harris et al., 1992). In Eurasian otters taken from the wild in Denmark and Norway, a single dark line is produced annually (Heggberget, 1984).
Here, we present the results of cementum banding analysis of otters Lutra lutra found dead in England and Wales between 1992 and 2004. We discuss the age structure of the animals found, and potential errors implicit in using cementum analysis to age Lutra lutra from the UK.
ANIMALS, MATERIALS AND METHODS
Sample collection
Since 1992, otters found dead in England and Wales have been sent for post mortem examination to Cardiff University. During examination, data are collated following a standard protocol (www.otterproject.cf.ac.uk) including sex and age-class. Otters are classified as juvenile where body weight is <2.1kg (females) or < 3kg (males). Where these weights are exceeded, individuals are categorised as adult if reproductively mature (baculum length >60mm [males], signs of uterine thickening, placental scarring, previous or current lactation, or pregnancy [females]), or as sub-adults if reproductively immature.
Following post mortem examination, skulls are retained and cleaned. For this study, teeth were taken from 143 individuals (87 male, 56 female), for cementum analysis. Newly erupted teeth (those with a thin walled root and a large apical foramen) were counted, but omitted from cementum analysis. For carnivores, it is recommended to use lower canines for cementum analysis (Matson, 1981). Here, the lower left canine was taken where available (n = 131) or the lower right where the left was not available (n = 12). In order to assess reproducibility of results for left and right canines, and for different tooth types, groups of teeth from the same individual were taken as follows; upper left incisor, left canine, and right canine (n = 21), lower left canine and lower right canine (n = 3).
Cementum analysis
Tooth preparation and cementum analysis was undertaken by Matson’s Laboratory, USA, following their standard procedure (http://www.matsonslab.com). A standardized analysis model specific to otters and the tooth type was then applied to count annuli. Matson’s Laboratory based the age model for Eurasian otters on that of North American river otters (Lontra canadensis)so assume dark cementum rings are deposited in winter and assume a birth date of April. A reliability grading was given to each tooth, ‘A’ where histology was good, ‘B’ where it was reasonable. ‘X’ was given where ageing was not attempted due to cementum damage.
Data analysis
Preliminary analysis was carried out to test whether the subset of cementum aged animals was representative of the post mortem dataset as a whole (n = 515 [males], n = 348 [females] to June 2008). Otters are sexually dimorphic so sexes were analysed separately. Juveniles were eliminated from the comparison because newly erupted teeth were excluded from the aged data set. Analysis of Covariance using a General Linear Model was applied to test whether the aged animals differed from the non-aged animals in terms of animal size (weight, total length, tail length, femur, tibia and fibula length and weight) and condition (Wt/L), and showed no significant difference.
Analysis of Covariance using a General Linear Model was applied to test whether there were differences in the age structure of the male and female populations. All analyses were performed using Minitab 15.
RESULTS
Age
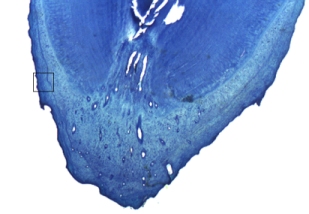
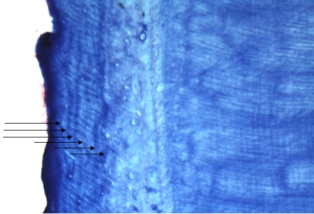
Ageing was not possible for 45 teeth, representing 33 individuals (9 females and 29 males). This was due to poor histology, resulting from the use of detergents containing bleach to clean the teeth, prior to the decision to perform cementum analysis. Histological sections were good (graded A or B) for 62 male and 48 female otters (e.g. Figure 1); data from these were used in further analysis.
 |
 |
| A | B |
| Figure
1. Cementum banding in Eurasian otter aged 6; A – root of otter tooth; B – enlarged version of cementum showing 6 (discontinuous) annuli
(click images for larger versions) |
|
Reproducibility of cementum ageing for different canines from the same animals (n = 19 pairs) was 78.95% (fifteen aged the same, three cases with a one year difference, and one case with a two year difference). Matched pairs of incisors and canines (n = 14) showed a lower reproducibility of 57.1% (eight were aged the same, four aged one year different, and two aged two years different).
The age distribution was skewed towards younger animals, with the majority of otters aged between 0 and 2 years. Only 10 otters were 4 years or older, and the maximum age recorded was 8 yrs (Figure 2). There was no significant difference between the age structures of males and females (F ratio = 0.76, df = 1, P = 0.398).
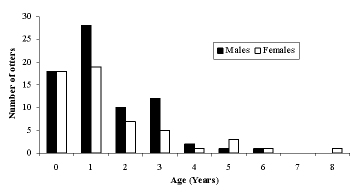 |
| Figure 2. Age distribution of otters (click for larger version) |
DISCUSSION
Our data suggest a skewed age distribution, with most otters being less than three years of age, and the most frequent age being just one year old. There was no significant difference in age distribution between males and females. A similarly young age structure was found in Shetland, where otters were most commonly recorded in their first year (Kruuk, 2006). In Germany, the most frequent age class was again 1 year old, but otters aged 2-10 were also frequently found, and the maximum age was 16 years (from a sample size of 225) (Ansorge et al., 1997). Hauer et al.(2002a) also found many otters in age classes 3, 4-9 and >9 years (maximum age found is not given).
Interestingly, Hauer et al. (2002b) found the bulk of reproductively active females in Germany were 6-9 years of age. In our study we had only two females aged 6 or over. This suggests either that the age distribution presented here does not mirror that of the actual population, females breed at an earlier age in England and Wales than those in Germany, or that many otters are killed prior to peak reproductive age in England and Wales.
The majority of otters in this study were killed by traffic (94%). This is likely to be biased, because road kills are more detectable than natural deaths. However, this represents one of the highest proportions in Europe and can be compared to 36.1% in Norway (Heggberget, 1991); 69.9% in Germany (Hauer et al.,2002a); 64% in Upper Lusatia, Germany (Ansorge et al., 1997) and 83% in south-west England (Simpson, 1997). It is possible that the age structure differs between our sampled population and the true population. Hauer (2002a) found that among otters sampled from road traffic mortalities, the most common age was 3 years, whereas among otters killed by disease or by hunting both young (<1) and old (>9) otters were more commonly found than intermediate ages. We have insufficient data for non road traffic mortalities to make a similar comparison.
In addition to potential bias associated with sample collection, it is also important to recognise problems associated with cementum analysis. In a study of 10 known-age captive otters, 1-11 years old, Yom-Tov et al.(2006) described the annuli as irregular and discontinuous, and maximum counts underestimated age by a year in half the animals examined. This may be because animals in captivity are less exposed to seasonal variation in climate and diet, but for wild North American river otters Lontra canadensis banding patterns are also described as indistinct, with peripheral compression of annual bands causing difficulty in ageing (Matson, 1981). In our study, ageing was not possible for 23% of individuals, but this failure rate was largely due to unsuitable cleaning protocols used at a time when cementum analysis was not planned.
Where histology is clear and counts can be made, interpretation to infer age remains subject to further error. Interpretation relies on two assumptions; an assumed season of band formation, and an assumed birth date. In most analyses it is assumed that a thin dark band of cementum forms in winter, and a broader, lighter band forms during the growth seasons of spring and summer (Matson, 1981). Grue and Jensen (1978) found that in Danish otters (Lutra lutra) incremental lines develop in winter-spring. In analyses of otter teeth from Norway, Heggberget (1984) found signs of dark-staining cementum in the process of deposition in all months from February to July, suggesting that band formation is variable. Here, we assume winter band formation, but known-age animals have not been used to test this in the UK, and error in this assumption could cause errors in ageing.
If we do assume winter band formation, the number of dark bands equates to the number of winters an otter has experienced since eruption of the adult teeth. To reach a more accurate assessment of age it is necessary to add the number of months survived prior to first band formation, and following the last band. The latter can be readily estimated using time elapsed between the most recent winter and the month of death (recorded when the animal was found). The former is a summation of two periods; (i) the period between birth and eruption of the adult teeth (in Eurasian otters, ca. 5 - 5.5 months (Heggberget, 1996)), and (ii) the period between eruption of the adult teeth and the first band-producing winter. Here, an assumed birth month must be used. In species or areas where reproduction is seasonal, the number of months between eruption of the adult teeth and the first winter can be readily estimated. For example, if an animal was found dead in April 2009, and one band was recorded, we would assume that it was born the previous April (2008), allowing adult teeth to develop by September-October 2008, and the first band to form in winter 2008-09. However, if birth date is unknown, the estimated age varies: it could have been born as late as June 2008 (adult teeth formed early winter 2008-09, band formation that same winter, at 6 months old, and died four months later), or as early as July 2007 (adult teeth not developed until mid-winter 2007-08 therefore not banded until its second winter, at 17 months old, and died four months later). Here, age would be recorded as one year, but the actual age might be anything from 10 to 21 months, i.e. the given age could be a 2 month overestimate or 9 month underestimate.
Seasonality of reproduction in Eurasian otters has been reported in some areas, for example Kruuk et al. (1987) observed a breeding season for Shetland otters in June, whilst Heggberget and Christensen (1994) described a late summer-autumn breeding season for otters in Norway. However in England and Wales it is generally stated that reproduction can occur in any month of the year, with no distinct seasonality (Mason and MacDonald, 1986). Stephens (1957) reports estimated dates of birth for 134 reliable instances on which cubs were sighted spread evenly throughout the year. Data collected by CUOP also shows no evidence of seasonality in reproduction, with pregnant females found throughout the year (Chadwick and Sherrard-Smith, 2010). Given the lack of reproductive seasonality among our sample population, the potential errors associated with using an assumed birth month may be considerable.
When calculating the potential errors associated with using an assumed April birth month, birth in January-March or July-December lead to an underestimate of age, while birth dates in May or June lead to an overestimate of age. Otter age is therefore more likely to be under- than over-estimated. Where, as in this study, the majority of animals are aged at <2 years old, a potential 9 month error in age may be problematic; using morphometry, reproductive status and observations such as tooth wear may give a more biologically relevant estimate of age than cementum ageing.
Acknowledgements - Thank you to the Environment Agency for funding the Cardiff University Otter Project. Thank you also to Eleanor Kean of her contributions and support. Thank you for the translation of the French and Spanish abstracts by Muriel Alix and Sonia Valladares respectively.
REFERENCES
Ansorge, H., Schipke, R., Zinke, O. (1997) Population structure of the otter, Lutra lutra: parameters and model for a central European region. Z. Säugetierkd. 62: 143-151.
Bodkin, J.L., Ames, J.A., Jameson, R.J., Johnson, A.M. and Matson, G.M. (1997) Estimating age of sea otters with cementum layers in the first premolar. J. Wildlife Manage. 61(3): 967-973.
Brothwell, D.R. (1989) The Relationship of Tooth Wear to Ageing. In: Iscan, M Y (ed.) Age Markers in the Human Skeleton. Springfield: Charles C Thomas.
Cardiff University Otter Project: www.otterproject.cf.ac.uk
Castanet, J., Francillon-Vieillot, H., Meunier, F.J., De Ricqles, A., (1990) Bone and Individual Aging. In: Hall, B K (ed.) Bone. Vol. 7
Chadwick, E.A., Sherrard-Smith, E. (2010) Pregnancy among otters (Lutra lutra) found dead in England and Wales: foetal development and lack of seasonality. IUCN Otter Specialist Bulletin, 27 (1): 33 - 41
Grue, H. and Jensen, B. (1978) Review of the formation of incremental lines in tooth cementum of terrestrial mammals. Danish Review of Game Biology 11(3): 3-48.
Harris, S., Cresswell, W.J. and Cheeseman, C.L. (1992) Age-determination of badgers (Meles meles) from tooth wear - the need for a pragmatic approach. J. Zool. 228: 679-684.
Hauer, S., Ansorge, H., Zinke, O. (2002a) Mortality patterns of otters (Lutra lutra) from eastern Germany. J. Zool.256: 361-368.
Hauer, S., Ansorge, H., Zinke, O. (2002b) Reproductive performance of otters Lutra lutra (Linnaeus, 1758) in Eastern Germany: low reproduction in a long-term strategy. Biol. J. Linn. Soc. 77: 329-340.
Heggberget, T.M. (1984) Age determination in the European otter, Lutra lutra lutra. Z. Säugetierkd. 49: 299-305.
Heggberget, T.M. (1991) Sex and age distribution in Eurasian otters (Lutra lutra) killed by human activities. In: Reuther, C., Röchert, R. (eds.) Proceedings of the Vth International Otter Colloquium. Habitat 6: 123-125.
Heggberget T.M. (1996) Age determination of Eurasian otter (Lutra lutra L.) cubs. Fauna Norvegica 17: 30-32.
Heggberget, T.M., Christensen, H. (1994) Reproductive Timing in Eurasian Otters on the Coast of Norway. Ecography 17(4): 339-348.
King, C.M. (1991) A Review of Age-Determination Methods for the Stoat Mustela-Erminea. Mammal Rev. 21(1): 31-49.
Kruuk, H., Conroy, J.W.H. and Moorhouse, A. (1987) Seasonal reproduction, mortality and food of otters (Lutra lutra ) in Shetland. Sym. Zool. S. 58: 263-278.
Kruuk, H. (2006) Otters: Ecology, behaviour and conservation. Oxford University Press.
Lieberman, D.E. (1994) The Biological Basis for Seasonal Increments in Dental Cementum and Their Application to Archaeological Research. J. Archaeol. Sci. 21(4): 525-539.
Mason, C.F. and Macdonald, S.M. (1986) Otters: Ecology and Conservation. Cambridge, UK: Cambridge University Press.
Matson, G.M. (1981) Workbook for Cementum Analysis. Milltown, Montana: Matson's Milltown.
Matson’s Laboratory: Cementum and Aging Wildlife Services: http://www.matsonslab.com/:
Moffit, S.A. (1998) Aging Bison by the Incremental Cementum Growth Layers in Teeth. J. Wildlife Manage. 62(4): 1276-1280.
Norgaard, N. and Larsen, B.H. (1991) Age determination of harbour seals by cementum growth layers, X-ray of teeth and body length. Danish Review of Game Biology 14(4): 17-32.
Simpson, V.R. (1997).Health status of otters (Lutra lutra) in south-west England based on post-mortem findings. Vet. Rec. 141: 191-197.
Stephens, M.N. (1957) The Otter Report. The Universities Federation for Animal Welfare, Potters Bar, Herts.
Stoneberg, R.P., Jonkel, C.J. (1966) Age Determination of Black Bears by Cementum Layers. J. Wildlife Manage. 30(2): 411-414.
Tanaka, R. (1956) On differential response to life traps of marked and unmarked populations. Annals of Zoology, Japan 29: 44-51.
Teska, W.R., Pinder, J.E. (1986) Effects of nutrition on age determination using eyelens weight. Growth 50: 362-370.
Yom-Tov, Y., Heggberget, T.M., Wiig, O. and Yom-Tov, S. (2006) Body size changes among otters, Lutra lutra, in Norway: the possible effects of food availability and global warming. Oecologia 150(1): 155-160
Résumé :
L’âge est un paramètre important pour comprendre la structure d’une population ainsi que les procédés dépendant de l’âge comme l’accumulation de contaminants.
Dans cette étude, les canines et incisives, prélevées chez des subadultes et adultes de loutres sauvage (Lutra lutra) de populations anglaises et galloises, ainsi que les céments dentaires, ont permis de déterminer l’âge des individus. La structure de l’âge de cet échantillon de population est beaucoup plus jeune que pour certaines populations européennes (sur 110 individus, seulement 10 étaient âgés de 4 ans ou plus).
Le cément dentaire donne une approximation de la strucutre de l’âge mais n’est pas assez précis pour des populations qui ne montrent pas de période de reproduction saisonnière.
Revenez au dessus
Resumen:
La edad es un parámetro importante para entender la estructura de una población y los procesos dependientes de la edad como son la acumulación de contaminantes. Los caninos e incisivos de individuos subadultos y adultos de nutrias salvajes (Lutra lutra) de Inglaterra y Gales fueron examinados por las líneas de incremento del cemento, las cuales son utilizadas como un indicador de la edad en muchas especies de mamíferos. La estructura de edad de la población de muestra es mucho más joven que algunas poblaciones europeas (de 110 nutrias estudiadas, sólo 10 de ellas tenían 4 años o más). El envejecimiento del cemento dental aquí es útil para obtener una amplia visión de la estructura de edad, pero es imprecisa para especies que no exhiben una reproducción estacional. La edad es probable que sea subestimada en muchos casos.
Vuelva a la tapa