IUCN/SSC Otter Specialist Group Bulletin

|
©IUCN/SCC Otter Specialist Group Volume 29 Issue 2 Pages 70 - 120 (June 2012) Citation: Rheingantz M.L., Oliveira-Santos, L.G., Waldemarin, H.F. and Pellegrini Caramaschi, E. (2012). Are Otters Generalists or do they prefer Larger, Slower Prey? Feeding Flexibility of the Neotropical Otter Lontra longicaudis in the Atlantic Forest . IUCN Otter Spec. Group Bull. 29 (2): 80 - 94 Are Otters Generalists or do they prefer Larger, Slower Prey? Feeding Flexibility of the Neotropical Otter Lontra longicaudis in the Atlantic Forest Marcelo Lopes Rheingantz1 , Luiz Gustavo Oliveira-Santos1 , Helen Francine Waldemarin2, and Erica Pellegrini Caramaschi3
1Laboratório de Ecologia e Conservação de Populações. Av. Carlos Chagas Filho, 373. CCS / Instituto de Biologia. Departamento de Ecologia, Cx. P. 68020. Universidade Federal do Rio de Janeiro. Rio de Janeiro, RJ, 21941-590. Brazil. e-mail:
mlrheingantz@gmail.com
|




|
| Received 17th April 2012, accepted 23th June 2012 |
| Abstract: Despite there being several studies focusing on feeding habits of Lontra longicaudis, few studies aimed to evaluate its prey selectivity and none of them considered prey mobility. In this study, we report both its feeding flexibility and specialist feeding behaviour between two parts of Mambucaba River, Southeastern Brazil. We observed that they fed mainly on fish, crabs and crayfish. We did not observe seasonality either in diet or prey community availability. However, using ANOVA, we found differences between stretches for diet composition and in the availability of prey. Monotonic Multi-Dimensional Scaling ordination showed that the otter diet in mangroves was dominated by Brachyura and the prey availability by Brachyura, Caridea, Ariidae, Mugilidae, Gerreidae, Centropomidae and Cichlidae, while the diet in the river stretch was dominated by Cichlidae, Caridea and Heptapteridae, and the prey availability by Characidae, Erythrinidae and Heptapteridae. According to Ivlev Electivity Index, along the river few preys were consumed according to their abundance, the majority being selected. Otters preferred slower prey, no matter their size. We observed variation in the level of preference of the same prey in different stretches, with flexibility in otter diet. Otter ate few preys according to their abundance, but showed specialist feeding behaviour, eating the slowest prey of the stretch. |
| Keywords: Brazil; diet; Lontra longicaudis; optimal foraging; prey preference |
| Française | Español | Português |
INTRODUCTION
The Neotropical otter Lontra longicaudis (Olfiers 1818) is a top predator in aquatic environments, and has a widespread distribution in Latin America, from Central Argentina to Mexico ( Eisenberg and Redford 1999 ). Despite its wide distribution, it is one of the less studied otter species, with most of the work being done on distribution ( Astúa et al., 2010 ), use of shelters ( Pardini and Trajano, 1999 ), or feeding habits ( Pardini, 1998 ; Quadros and Monteiro-Filho, 2001 ; Rheingantz et al., 2011 ). Neotropical otters feed mainly on fish, with crustaceans as the second main prey item, but few studies have identified the relative importance of each prey species in the diet ( Pardini, 1998 ; Quadros and Monteiro-Filho, 2001 ; Gori et al., 2003).
Otter populations seem to be influenced by the stock of available food resources ( Ostfeld, 1982 ; Kruuk and Moorhouse, 1990 ; Carss and Kruuk 1996 ). However, according to various studies, diet composition of otters does not always reflect total prey abundance in the environment, which suggests that otters have feeding preferences ( van der Zee, 1981 ; Wise et al., 1981 ; Kruuk and Moorhouse, 1990 ; Kruuk, 1995 ; Pardini, 1998 ; Quadros and Monteiro-Filho, 2001 ; Kasper et al., 2004 ). On the other hand, several studies have suggested that, when considering the prey available for otters in the aquatic environment, they might feed mainly on animals with greatest abundance or on those with habits facilitating their predation (e.g. low mobility, solitary) ( Erlinge, 1968 ; Adrian and Delibes, 1987 ; Tumlison and Karnes, 1987 ; Weber, 1990 ; Cote et al., 2008 ).
The predator’s decision whether to attack their prey or not depends on whether the foraging time and the energy spent capturing the prey is compensated for or exceeded by the prey’s energy content ( Charnov, 1976 ; Pianka, 2000 ). However, the predator can choose not to eat a prey that is easy to catch but having low energy content, preferring to continue searching for higher quality food ( Krebs and Davies, 1987 ). Considering that prey availability and the time needed to find the prey varies, the predator’s decision must involve balancing a cost-benefit relationship to maximize its chances of survival ( Krebs and Davies, 1987 ). The prey availability concept integrates notions of prey abundance, prey concealment and prey capture success once detected ( Johnson, 1980 ), but we concentrated our discussion in this study only on prey abundance, as the other measures were not available, being very difficult to establish in our field conditions. However, despite lacking prey behaviour information, this field study may still lead to relevant insights on variables that affect prey selection by predators ( Charnov, 1976 ).
Based on optimum foraging theory, we predicted that Neotropical otters are generalists, feeding on preys according to their abundance from mangrove to river, in both wet and dry seasons, without seasonality. If Neotropical otters actively select certain prey, we predicted that they prefer less mobile, larger prey, avoiding highly mobile, smaller prey. Thus, we believed that larger prey would have higher energy content and cost less energy to catch, and so would be present in the diet in higher proportion than their actual occurrence in the environment.
The present study aimed to identify the main preys of the Neotropical otter in the lower Mambucaba river catchment (Rio de Janeiro state, Brazil), including seasonal and spatial differences in the feeding behaviour of the species, in relation to aquatic prey availability in the same catchment, and to analyze prey selectivity by Neotropical otters.
MATERIAL AND METHODS
Study area
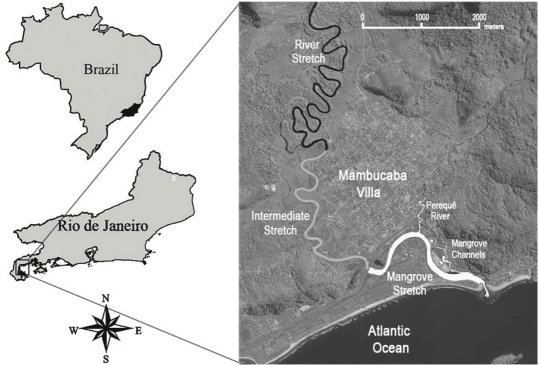
The present study was conducted in Angra dos Reis municipality, including the last 13km of the Mambucaba River, 1km of the Perequê river (tributary of Mambucaba River), and 1km of nearby swamp channels at the outlet of the Mambucaba River in Ilha Grande Bay; a typical coastal Atlantic Forest environment ( Fig. 1 ).
The weather is hot and wet, with marked seasonality. Annual precipitation is approximately 2.240 mm, with maximal rainfall occurring in January (75mm) (rainfall data obtained by Meteorology System of Nuclear Central Almirante Álvaro Alberto). The temperature is about 23.2 ºC, with the highest temperature in February (25.3 ºC) and July is the coldest month, with 20 ºC ( Natrontec, 1998 ). The study area, in the lower Mambucaba River (between 23º01’40,61’’ S, 44º 31’12,02’’ W and 22º59’16,50’’ S, 44º32’33,88’’ W) ( Fig. 1 ), has low water flow and a high level of anthropogenic influence. A national roadway crosses the river, and there is deforestation, houses, sand extraction, and sewage flowing into the river ( Natrontec, 1998 ).
The stretch of Perequê River studied consists in its lower part, without riparian vegetation, and with sewer discharge. The Mangrove channels, on the other hand, show good mangrove vegetation and no more sewer discharges.
We divided the study area in three stretches (5 km each) along the river, in coast-continent direction:
- Mangrove stretch: this diversified stretch includes the lower stretch of Perequê River, a tributary of Mambucaba River, and mangrove channels. It is close to the coast, with saline water (between 0 to 18 ppt), and tidal (50-100 cm) influences. The bottom is composed of mud and sand, and the water has the highest temperatures (21.5-24.1 ºC).
- Intermediate stretch (3-8 km from the river’s mouth): this section has freshwater and does not have tidal influence. It has higher water flow, depth (3.2-5.5 meters), and water transparency (>1m), all the while having lower conductivity (36.9-132 μS) and water temperature (18.5-22.1 ºC) than the mangrove stretch. The riverbed is dominated by sand, with few rocks and some fallen trees.
- River stretch: located in the upper part of the study area (9-13 km from the river’s mouth), has large rocks and many of fallen trees, with a gravel-sand riverbed. This stretch has the lowest values of conductivity (27.8-67.1 μS) and water temperature (17.5-21.3 ºC).
Neotropical otter diet
We carried out six spraint sampling sessions from October 2004 to April 2006, three per season (dry and rainy). In each spraint sampling, we traveled the chosen river stretch by boat, going up the river along one margin and down the river on the other, collecting all the spraints found. Otters usually defecate in conspicuous places such as riverbanks, large rocks in the river, or latrines found on river beaches ( Kruuk 2006 ). We packed each spraint found into a plastic bag and labeled it with stretch and season information. We then later placed the spraint on a sieve (1 mm net) and washed it in flowing water. After washing, we dried each sample in an oven (40 ºC) for 48 hours, separating the remaining prey content in taxonomic groups, with the help of ichthyologists, (fish down to family level), carcinologists (classifying as crabs or crayfish) and reference collections. Fish were identified mainly by scales (when they have unique scale format), head bones and vertebrae and the crustaceans were identified by hard pieces, legs and chelas. Diet composition was described in frequency of occurrence of the spraint items: number of spraints in which each prey type occurs divided by the total number of spraints ( Erlinge, 1968 ).
Prey availability
We estimated the relative abundance of fish, crayfish and crab species during six prey sampling sessions concomitant with the six otter spraint sampling sessions. Fish abundance was estimated in each stretch using a standardized gillnet, composed of five nets (15, 20, 25, 30 and 40 mm between knots, each net was 15m2) for 24 hours. The fish were identified to species level and the species’ relative abundance was measured in number of individuals per square meters per hour (Catch Per Unit Effort). The crayfish abundance was estimated in each stretch using 10 crayfish pots (2l. PET bottles without bottle cover cut into its middle. The top part is turned and inserted inside the other). Crayfish were identified to species level and the relative abundance was measured as number of individuals per pot per hour. Crab abundance was sampled, in each stretch, by active searching carried by two observers during 15 minutes. To avoid biased abundance estimates between stretches’ differences in habitat structure that could cause heterogeneity in crabs’ detection, this active searching was carried on the stretch margins, where we found similar visible conditions. The crabs were identified to species level and the species’ relative abundance in each sample was measured as the number of individuals collected per sampling session.
Data analyses
In our analyses we used all prey items (fish, crayfish and crabs) to test our predictions, assuming that the energetic rewards of these items is proportional to their size. This assumption is supported by bioenergetic and physiological studies showing that the calorific value (CF) and digestibility (D) of crustaceans (CF ~ 4.1 kJ g-1 and D = 50-60%) are similar to fish (CF ~ 4.5 kJ g-1 and D = 70%) (see details in Prus, 1970 ; Cummins and Wuycheck, 1971 ; Costa, 1982 ). Since these values were taken in temperate areas where fish are fatter than tropical fish, this assumption might be even more valid in our study because Neotropical fish probably have calorific value and digestibility even more similar to crustaceans.
We used Monotonic Multi-Dimensional Scaling (MMDS) ordination to reduce the dimensionality of the prey items found in each spraint, from a Jaccard similarity matrix. Prey composition was defined as the presence or absence of each prey item in each spraint. We assumed that fish bones and scales, and crab and crayfish exoskeletons have a similar chance to be found in the otter spraint once eaten by the animal. Although this assumption was not tested, it seems to be realistic, because all these prey have large proportion of indigestible parts.
We checked whether the spraint composition (MMDS dimension – dependent variable) varied depending on the stretch and season factors, using two-way analysis of variance (ANOVA) ( Zar, 1999 ). For analysis, we assumed that the prey items found in otter spraints in a certain stretch represented the diet of the Neotropical otter in that stretch. The fish found in the otter spraints were classified just to family level and the crabs and crayfish were classified to infra-order level, so we pooled the prey abundance data into the same categories groups used for otter spraints, to permit comparisons.
We also used MMDS ordination to reduce the dimensionality of the availability of prey in each prey sampling session from a Bray-Curtis similarity matrix. We defined the relative frequency of the various species found as the availability of prey. As we used different sampling methods for different prey (fish, crayfish and crabs), generating different scales of abundance measures between prey, we standardized the abundance of each prey item. The standardization was done by dividing the abundance of each prey, in each sampling stretch, by the total sum of the abundances within this prey, before generating the similarity matrix. This procedure standardized potential differences in abundance among the main prey groups (fish, crayfish and crabs) that were sampled using different methods and scales, and permitted the abundance comparison among these groups. We checked if the availability of prey (MMDS dimension - dependent variable) varied depending on the stretch and season factors, using two-way analysis of variance (ANOVA) ( Zar, 1999 ).
The generalist behaviour of otters was checked graphically, identifying if the more frequent prey in the environment for each stretch, within each season, were more abundant in the otter spraints for the same stretch and season. We characterized the otter preferences for each prey, in each stretch, within each season, using the Ivlev Electivity Index (IEI) ( Krebs, 1998 ) adjusted to a symmetrical output with respect to zero (see Reynolds-Hogland and Mitchell, 2007 ). Values close to -1 indicated that the otter rejected the prey, values close to 1 indicated that otters preferred the prey and values around 0 indicated that the prey was consumed according to its abundance. Although the approach based on frequency of occurrence is assumed to under or overestimate some prey in the diet, we believe that these problems were decreased in our analysis, once we supposed that fish scales or bones, crab and crayfish exoskeletons have similar likelihood of being found in the otter spraint once eaten by an otter. We acknowledge that this assumption was not tested here, but it seems to be realistic, since all these prey have a large proportion of indigestible parts. Furthermore, Jacobsen and Hansen (1996) and Perini et al.(2009) show elegantly, in controlled studies with captive otters, that the frequency of occurrence could retrieve about 80% of the real diet offered to an individual otter, enabling us to use this measure in our analysis. For each stretch within each season, we checked if otters preferred (IEI – dependent variable) prey with low mobility and a larger size using analysis of covariance (factor: prey mobility; co-variable: prey size) (ANCOVA) ( Zar, 1999 ). The mobility of each prey species was classified as fast or slow-sedentary, according to its behaviour, with specialist help. We could not measure this variable in a continuous scale (e.g. velocity) because they require controlled laboratory experiments. On the other hand, we believe that our coarse classification holds ecological meaning, and that it is an adequate way to test the mobility effect on otter prey preferences. Prey size was measured (length in cm) based on specimens collected during the prey sampling. Fish were measured by standard length, as were crayfish, and crabs were measured by shell length. All analyses were performed in Systat 11.0 (Systat software, Inc., 2004).
RESULTS
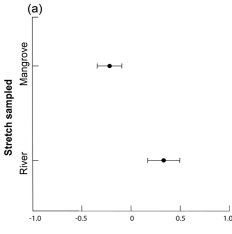
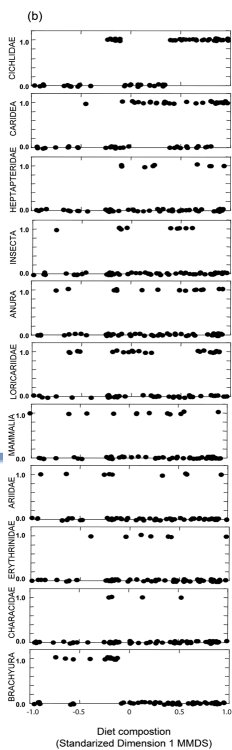
We examined 105 spraints in the Mambucaba River watershed. Fish (mainly Cichlidae), crayfish, crabs and amphibians were the most abundant food items found ( Table 1 ). Since we collected in the intermediate stretch only two spraints during the dry season and only five spraints during the rainy season, we removed this stretch from the analysis. One dimension in MMDS ordination recovered 87% of the diet composition variation indicated in Jaccard Similarity Matrix (Stress = 0.12). We observed differences in diet composition when we compared mangrove and river stretches (two-way ANOVA; F1,94=3.39; P=0.03) ( Fig. 2a ). However, we did not observe seasonal differences (F1,94=0.03; P=0.87), nor interaction between the stretch and season factors (F1,94=0.04; P=0.96). Diet composition in the mangrove stretch was dominated by crabs (Brachyura), while in the river stretches, Cichlidae, Caridea, and Heptapteridae were more common ( Fig. 2b ). Insects, amphibians, reptiles, mammals and Characidae appear in the spraints along the entire study area ( Fig. 2b ).
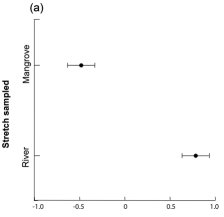
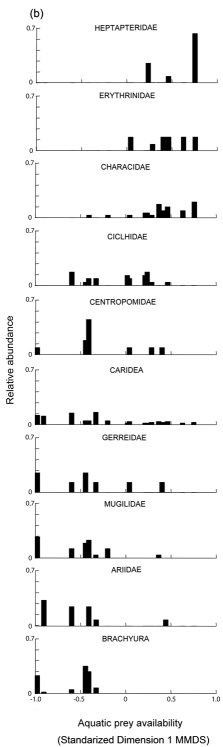
MMDS ordination in one dimension recovered 68% of the prey community variation indicated by Bray-Curtis similarity matrix (Stress = 0.22). We observed differences in prey availability between the mangrove and river stretches (two-way ANOVA; F1,8=21.837; P<0.001) ( Fig. 3a ), but we did not find differences between seasons (F1,8=0.624; P=0.45), nor interaction of stretch and season factors (F1,8=1.046; P=0.38). The prey availability in the mangrove stretch was dominated by crabs (Brachyura), crayfish (Caridea) and fish families Ariidae, Mugilidae, Gerreidae, Centropomidae, and Cichlidae ( Fig. 3b ), while the river stretch was dominated by fish of the families Characidae, Erythrinidae, and Heptapteridae.
Concerning the general longitudinal differences from mangrove to freshwater stretch, prey such as crabs (Brachyura) and fish of the family Heptapteridae were consumed along the stretches sampled according to their abundance ( Table 2 ; Fig. 2 , 3 ), while some fish families were consumed in different proportions than expected along the river ( Table 2 ; Fig. 2 , 3 ) and within each stretch (Table 2).
| Table 2:Neotropical Otter (Lontra longicaudis) prey preferences according the adjusted Ivlev Electivity Index (IEI) in each stretch sampled, the categorical prey mobility and the prey mean size. The preys used in this analysis were fishes, crabs and crayfishes. | ||||
| Prey | IEI | Mobility | Mean size (cm ± SD) |
|
|
|
||||
| Mangrove | River | |||
|
|
||||
| Ariidae | 0.033 | 0.000 | slow - sedentary | 18.0 ± 3.1 |
| Heptapteridae | 0.032 | 0.107 | slow - sedentary | 25.1 ± 3.3 |
| Sciaenidae | -0.212 | 0.000 | fast | 16.1 ± 1.0 |
| Mugilidae | -0.364 | 0.026 | fast | 19.2 ± 5.3 |
| Erythrinidae | 0.063 | 0.196 | slow - sedentary | 25.3 ± 7.4 |
| Characidae | 0.084 | -0.585 | fast | 14.2 ± 4.1 |
| Eleotridae | * | * | slow - sedentary | 7.7 ** |
| Poecilidae | * | * | slow - sedentary | 3.5 ** |
| Cichlidae | 0.501 | 0.486 | slow - sedentary | 13.7 ± 2.3 |
| Centropomidae | -0.177 | * | fast | 26.0 ± 6.0 |
| Gerreidae | -0.066 | * | fast | 11.7 ± 2.5 |
| Paralichtyidae | -0.033 | * | slow - sedentary | 3.4 ** |
| Belonidae | -0.033 | * | fast | 8.7 ** |
| Loricariidae | 0.092 | 0.526 | slow - sedentary | 13.1** |
| Brachyura | 0.156 | 0.250 | slow - sedentary | 7.8 ± 1.8 |
| Caridea | -0.194 | -0.049 | slow - sedentary | 6.6 ± 0.8 |
|
|
||||
|
* Rare species - IEI was not calculated because this index is not accurate to these species. **Standard deviation was not calculated because only one specimen was captured. |
||||
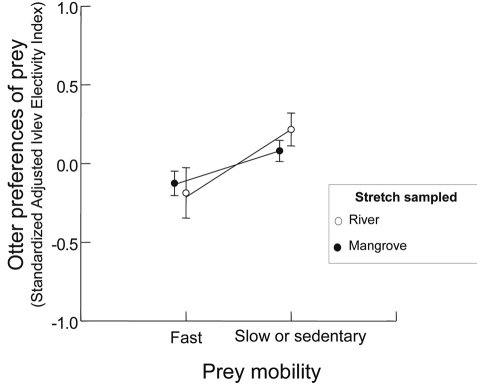
Since our results indicated that there was no seasonal effect on the otters’ diet composition and on the availability of prey, the seasonal data were grouped together in the analysis of prey preference. Slower prey were preferred in the mangrove stretch (two-way ANCOVA; F1,11=3.97, P<0.05), regardless of its size (F1,11=0.05, P=0.82) ( Figure 4 ). The same pattern was observed in the river stretches [two-way Ancova; (mobility effect; F1,7=4.42, P<0.05 and prey size effect; F1,7=0.001, P=0.99)] ( Figure 4 ). There was no interaction between mobility factors and size of prey in the stretches tested (mangrove stretch; F1,11=0.98, P=0.35 and river stretch; F1,7=0.26, P=0.63). There was no co-linearity between prey mobility and prey size factors, because fast and slow or sedentary prey showed similar sizes (t1,14 = 1.44; P=0.20).
DISCUSSION
The otters studied feed mainly on fish and crustaceans, as recorded in several previous studies on Lontra longicaudis ( José and Ker de Andrade, 1997 ; Pardini, 1998 ; Spinola and Vaughan, 1998 ; Colares and Waldemarin, 2000 ; Quadros and Monteiro-Filho, 2001 ; Gori et al., 2003 ; Rheingantz et al., 2011 ) as well as in other otter species ( Stenson et al., 1984 ; Adrian and Delibes, 1987 ; Roche et al., 1995 ; Clavero et al. 2003 , 2006 ; Cote et al., 2008 ). Freshwater otters’ diets usually show seasonal patterns ( Pardini, 1998 ), but in our study, we did not observe diet seasonality. The diet and prey availability were uniform through the seasons, similar to what was found for otters living in non-seasonal marine and coastal environments ( Kruuk et al., 1988 ; 1993 ; Cote et al., 2008 ).
Despite the fact that otters are usually described as generalist fish predators ( Kruuk and Moorhouse, 1990 , Breathnach and Fairley, 1993 , Carss, 1995 ), eating the most abundant items ( Jenkins and Harper, 1980 ; Tumlison and Karnes, 1987 ; Taastrom and Jacobsen, 1999 ), the present study shows that the Neotropical otter is capable of feeding on several available prey types, but the longitudinal variation observed in its diet was not equivalent to longitudinal variation in the prey abundance. In this way, some studies that analyzed prey abundance showed that otters select some kinds of prey or sizes ( Wise et al., 1981 ; Kruuk and Moorhouse, 1990 ; Pardini, 1998 ; Quadros and Monteiro-Filho, 2001 ). Our results suggest that otters can have an adaptive diet, similar to the findings of other studies focusing on different otter species ( Reid et al., 1994 ; Laidre and Jameson, 2006 ), feeding on a few prey species according to their abundance but mainly having a specialist feeding behaviour, preferring some items and avoiding others.
Laidre and Jameson (2006) also reported that sea otters Enhydra lutra (Fleming, 1822) changed their main prey when the first chosen was depleted, and suggested that when the sea otter densities approach population equilibrium, those animals diversify their diet, eating less profitable prey. Our results were similar to the observations of Stenson et al. (1984) with Nearctic otters Lontra canadensis (Schreber, 1777) and Wise et al. (1981) with Eurasian otters Lutra lutra (Linnaeus, 1758), which concluded that the fish most frequently eaten by the otters were those that were sluggish or fatigued faster, with a lower capacity for maintaining escape maneuvers. This suggests that otters capture the prey in direct proportion to its abundance and in inverse proportion of its escape ability.
In spite of the high feeding flexibility of the Neotropical otter, Pardini (1998) and Quadros and Monteiro-Filho (2001) suggested that it selects mainly bottom-dwelling and slow swimming prey. Similar patterns were suggested by other authors with different species of otters, predicting that otters feed mainly on prey with low escape ability ( Erlinge 1967 , 1968 ; Wise et al., 1981 ; Stenson et al., 1984 ), if this selectivity behaviour requires less energy for prey capture ( Pardini, 1998 ; Cote et al., 2008 ).
According to Roper (1994) and Quadros and Monteiro-Filho (2001) , a feeding specialist is an animal that has a diet based on few food items and the use of these items is not dependent on its abundance. This specialist behaviour can be predicted according to optimal foraging theory ( MacArthur and Pianka, 1966 ), where the predator will prefer to catch prey that increase the energetic return and decrease the energetic cost to catch them ( Krebs and Davies, 1987 ). This specialist feeding behaviour is widespread among carnivores ( Nowak et al., 2005 ) and normally the predator will prefer to catch larger and/or more vulnerable prey. On the other hand, a predator can choose to catch the “easier prey” that does not present toxic or mechanical defenses, group defense strategies, aggressive behaviour, or high escape ability ( Barbosa and Castellanos, 2005 ). In this trade-off, the Neotropical otters in our study preferred to catch the slower prey, no matter their size. Cote et al. (2008) have demonstrated that coastal-marine Nearctic otters selected both slower and larger prey. However, this selection of larger individuals was detected within each prey species, while our study tested preferences between prey species themselves.
Many fast fish were very abundant and reached low preference rank, while other slow-sedentary fish were rare and highly preferred. For instance, the fish families that were the most abundant in the mangrove stretch (Mugilidae) and the river stretch (Characidae), were the most avoided because they were represented by very fast species. Rare species in the mangrove stretch (Cichlidae) and in the river stretch (Erythrinidae) were preferred because they present slow-sedentary habits. Indeed, when we consider the longitudinal differences in the prey abundance in the assemblage, the same prey was avoided in one stretch and preferred in another. We hypothesize that the relative mobility ranking of each prey species depends on the prey community composition. For instance, one species could be the slowest in one stretch while in another stretch, there could be a prey species that is even less mobile and so would be the slowest, even when the first one was also present. This hypothesis could explain why some prey were preferred or avoided in one stretch and consumed according to its abundance in another, and why some prey were preferred in one stretch and avoided in the other one (see Mugilidae, Sciaenidae, Characidae and Loricariidae).
In this study, Neotropical otters presented high feeding flexibility, this being the first work to examine longitudinal variation in their diet, through mangrove to freshwater part of the river. The availability of prey was constant throughout the seasons and the diet composition was also constant. Neotropical otters consumed some prey according to their abundance, but they showed also a specialist feeding behaviour, preferring or avoiding several kinds of prey. Slower preys were preferred no matter their size, and the low frequency in the diet of faster prey could be expected due the high energetic cost to catch them. These results corroborate the hypothesis that the otters tend to forage optimally, looking to minimize the expense of energy in the capture of the prey, even where the result is less caloric earning per item, thus increasing the available energy to be allocated in, for example, reproduction (
Kruuk and Moorhouse, 1990
;
1991
;
Estes et al., 2003
).
Acknowledgements - We would like to thank "Hotel do Bosque" and “Associação Ecológica Ecomarapendi” for the logistic support, and CNPq, IdeaWild and Rheingantz family for financial support. We would like to thank Ademir, Henrique Lazarotto, Rafael Curcio and Marco Gonçalves for the help with the field work, all the colleagues of Laboratório de Ecologia e Conservação de Populações and Laboratório de Ecologia de Peixes, both of them in UFRJ, for the suggestions and for helping with statistical analysis, and Lesley Wright, Fernando Antonio dos Santos Fernandez, Thiago Queiroz, and Jordi Ruiz-Olmo for corrections in the text.
REFERENCES
Adrian, M.I., Delibes, M. (1987). Food habits of the otter (Lutra lutra) in two habitats of the Doñana National Park, SW Spain. J. Zool.
212: 399-406.
Astúa, D., Asfora, P.H., Aléssio, F.M., Langguth, A. (2010). On the occurrence of the Neotropical Otter (Lontra longicaudis) (Mammalia, Mustelidae) in Northeastern Brazil. Mammalia
74(2): 213-217.
Barbosa, P., Castellanos, I. (2005). Ecology of Predator-Prey Interactions. Oxford University Press. New York. USA. 416 pp.
Breathnach, S., Fairley, J.S. (1993). The diet of otters Lutra lutra (L.) in the Clare River system. Biol. Environ.93: 151-158.
Carss, D.N. (1995). Foraging behaviour and feeding ecology of the otter Lutra lutra: a selective review. Hystrix
7: 179-194.
Carss, D.N., Kruuk, H. (1996). Costs and benefits of fishing by a semi-aquatic carnivore, the otter Lutra lutra. In: Aquatic Predators and their Prey (S.P.R. Greenstreet and M.L. Tasker, eds.). Fishing News Books, Cambridge, pp. 10-16.
Charnov, E.L. (1976). Optimal foraging: Attack strategy of a mantid. Am. Nat.
110: 141-151.
Clavero, M., Prenda, J., Delibes, M. (2003). Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J. Biogeogr.
30: 761–769.
Clavero, M., Prenda, J., Delibes, M. (2006). Seasonal use of coastal resources by otters: Comparing sandy and rocky stretches. Est. Coast. Shelf S.
66: 387-394.
Colares, E.P., Waldemarin, H.F. (2000). Feeding of the neotropical river otter (Lontra longicaudis) in the costal region of the Rio Grande do Sul State, Southern Brazil.
IUCN Otter Spec. Group Bull. 17:6-13
Cote, D.,![]() Stewart, H.M.J. , Gregory, R.S., Gosse, J., Reynolds, J.J.,
Stewart, H.M.J. , Gregory, R.S., Gosse, J., Reynolds, J.J., ![]() Stenson and, G.B., Miller, E.H. (2008).
Stenson and, G.B., Miller, E.H. (2008).![]() Prey Selection by Marine-coastal River Otters (Lontra canadensis) in Newfoundland. J. Mammal.
86: 1001–1011.
Prey Selection by Marine-coastal River Otters (Lontra canadensis) in Newfoundland. J. Mammal.
86: 1001–1011.
Costa, D.P. (1982). Energy, nitrogen, and electrolyte flux and sea-water drinking in the sea otter Enhydra lutris. Physiol. Zool.
55: 35-44.
Cummins, K.W., Wuycheck, J.C. (1971). Caloric equivalents for investigations in ecological energetics. Mitt. Int. Ver. Theor. Angew. Limnol.
18: 1-158.
Eisenberg, J., Redford, K. (1999). Mammals of the Neotropics Volume 3, Chicago. USA. 624 pp.
Estes, J.A.![]() , Reidman
, Reidman![]() , M.L. , Staedler, M.M., Tinker
, M.L. , Staedler, M.M., Tinker![]() , M.T., Lyon, B.E. (2003). Individual variation in prey selection by sea otters: patterns, causes and implications. J. Anim. Ecol.
72: 44–155.
, M.T., Lyon, B.E. (2003). Individual variation in prey selection by sea otters: patterns, causes and implications. J. Anim. Ecol.
72: 44–155.
Erlinge, S. (1967). Food habits of the fish-otter (Lutra lutra L.) in south Swedish habitats. Viltrevy4: 371–443.
Erlinge, S. (1968). Food studies on captive otters Lutra lutra L. Oikos 19: 259-270.
Gori, M., Carpaneto, M.G., Ottino, P. (2003). Spatial distribution of the neotropical otter Lontra longicaudis in the Ibera Lake (northern Argentina). Acta Theriol.
48: 495-504.
Jacobsen, L., Hansen, H.M. (1996). Analysis of otter (Lutra lutra L.) spraints to estimate prey proportions: A comparison of methods through feeding experiment.
J. Zool. 238:
167–180.
Jenkins, D., Harper, R.J. (1980). Ecology of otters in Northern Scotland - II. Analyses of otter (Lutra lutra) and mink (Mustela vison) faeces from Deeside, N.E. Scotland in 1977-78. J. Anim. Ecol.
49: 737-754.
Johnson, D. (1980). Measurements for evaluating resource preference. Ecology 61: 65-71.
José, H., Ker de Andrade, H. (1997). Food and feeding habitats of the neotropical river otter Lontra longicaudis (Carnivora, Mustelidae). Mammalia
61: 193-203.
Kasper, C.B., Feldens, M.J., Salvi, J., Grillo, H.C.Z. (2004). Estudo preliminar sobre a ecologia de Lontra longicaudis (Olfers) (Carnivora, Mustelidae) no Vale do Taquari, Sul do Brasil. Rev. Bras. Zool.
21: 65-72.
Krebs, J.R., Davies, N.B. (1987). An introduction to behavioural ecology. Wiley-Blackwell. New Jersey. USA. 432 pp.
Krebs, C.J. (1998). Ecological methodology. Benjamin Cummings. New York. USA. 624 pp.
Kruuk, H. (1995). Wild otters: predation and populations. Oxford University Press. Great Britain. 290 p.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford University Press, New York. 280 pp.
Kruuk, H.![]() , Carss, D.N., Conroy, J.W.H., Durbin, L. (1993). Otter (Lutra lutra L.) numbers and fish productivity in rivers in north-east Scotland. Symp. Zool. Soc. Lond.
65: 171–191.
, Carss, D.N., Conroy, J.W.H., Durbin, L. (1993). Otter (Lutra lutra L.) numbers and fish productivity in rivers in north-east Scotland. Symp. Zool. Soc. Lond.
65: 171–191.
Kruuk, H., Moorhouse, A. (1990). Seasonal and spatial differences in food selection by otters (Lutra lutra) in Shetland. J. Zool.
221: 621-637.
Kruuk, H., Moorhouse, A. (1991). The spatial organization of otter (Lutra lutra) in Shetland. J. Zool.
224: 41-57.
Kruuk, H., Nolet, B., French, D.![]() (1988). Fluctuations in numbers and activity of inshore demersal fish in Shetland. J. Mar. Biol.
68: 601–617.
(1988). Fluctuations in numbers and activity of inshore demersal fish in Shetland. J. Mar. Biol.
68: 601–617.
Laidre, K.L., Jameson, R.J. (2006). Foraging patterns and prey selection in an increasing and expanding sea otter population. J. Mammal.
87: 799-807.
MacArthur, R. H., Pianka, E.R. (1966). On optimal use of a patchy environment. American Naturalist
100: 603-609.
Natrontec. (1998). Relatório de impacto ambiental (RIMA) da Usina de Angra II. Technical Report. Rio de Janeiro. Brazil.
Nowak, R.M., MacDonald, D.W.,Kays, R.W. (2005). Walker's Carnivores of the World. The Johns Hopkins University Press. Baltimore. USA. 328 pp.
Ostfeld, R.S. (1982). Foraging Strategies and Prey Switching in the California Sea Otter. Oecologia53: 170-178.
Pardini, R. (1998). Feeding ecology of the neotropical river otter, Lontra longicaudis, in an Atlantic Forest Stream, southeastern Brazil. J. Zool.
245: 385–391.
Pardini, R., Trajano, E. (1999). Use of shelters by the neotropical river otter (Lontra
longicaudis) in an Atlantic forest stream, southeastern Brazil. J. Mammal.
80: 600-610.
Perini, A. A., Vieira, E. M., Schulz, U. H. (2009). Evaluation of methods used for diet analysis of the neotropical otter Lontra longicaudis (Carnivore, Mustelidae) based on spraints. Mammal. Biol.
74: 232-237.
Pianka, E. R. (2000). Evolutionary ecology. 6th edition. Addison Wesley Longman, San Francisco. 512 pp.
Prus, T. (1970). Calorific value of animals as an element of bioenergetical investigations. Pol. Archs of Hydrobiol.17: 183-199.
Quadros, J., Monteiro-Filho, E. (2001). Diet of the neotropical otter, Lontra longicaudis, in an Atlantic Forest Area, Santa Catarina State, Southern Brazil. Stud. Neotrop. Fauna Environ.
36: 15-21.
Reid, D.G., Code, T.E., Reid, A.C.H., Herrero, S.M. (1994). Food habits of the river otter in a boreal ecosystem. Can. J. Zool.
72: 1306-1313.
Reynolds-Hogland, M.J., Mitchell, M.S. (2007). Effects of roads on habitat quality for bears in the Southern Appalachians: a long-term study. J. Mammal.
88: 1050–1061.
Rheingantz, M.L., Waldemarin, H.F., Rodrigues, L., Moulton, T.P. (2011). Seasonal and spatial differences in feeding habits of the neotropical otter Lontra longicaudis (Carnivora: Mustelidae) in a coastal catchment of southeastern Brazil. Zoologia 28: 37–44.
Roche K., Harris, R., Warrington, S., Copp, G.H. (1995). Home range and diet of re-introduced European otters Lutra lutra (L.) in Hertfordshire rivers. Aquat. Conserv. Mar. Freshwat. Ecosyst.
5: 87–96.
Roper, T.J. (1994). The European badger Meles meles: Food specialist or generalist? J. Zool.
234: 437-452.
Spinola, M.R., Vaughan, C. (1998). The diet of the neotropical otter (Lontra longicaudis) in Costa Rica.
IUCN Otter Specialist. Group Bull.
19(A: 341-345
Stenson, G.B., Badgero, G.A., Fisher, H.D. (1984). Food habits of the river otter Lutra canadensis in the marine environment of British Columbia. Can. J. Zool.
62: 88-91.
Taastrom, H.M., Jacobsen, L. (1999). The diets of otters (Lutra lutra L.) in Danish freshwater habitats: comparisons of prey fish populations. J. Zool.
248: 1-13.
Tumlison, R., Karnes, M. (1987). Seasonal changes in food habits of river otters in southwestern Arkansas beaver swamps. Mammalia
51: 225-232.
van der Zee, D. (1981). Prey of the Cape clawless otter (Aonyx capensis) in the Tsitsikama Coastal National Park, South Africa. J. Zool.
194: 467–483.
Weber, J.M. (1990). Seasonal exploitation of amphibians by otters (Lutra lutra) in north-east Scotland. J. Zool. 220: 641–651.
Wise, M.H., Linn, I.J., Kennedy, C.R. (1981). A comparison of the feeding biology of mink Mustela vison and otter Lutra lutra. J. Zool.
195: 181–213.
Zar, J.H. (1999). Biostatistical analysis. 4th ed. Prentice-Hall, New Jersey. USA. 663 pp.
Résumé : Les Loutres sont-elles Generalistes ou preferent-elles les Proies plus Grosses et plus Lentes? Flexibilite Alimentaire de la Loutre a Longue Queue Lontra longicaudis en Foret Atlantique
Bien qu'il existe plusieurs études portant sur les habitudes alimentaires de Lontra longicaudis, peu d'études visent à évaluer la sélectivité des proies et aucune d'entre elles ne considère la mobilité de ces proies. Dans cette étude, nous rapportons à la fois sa souplesse alimentaire et son comportement plus spécialisé entre deux zones de de la rivière Mambucaba au sud-est du Brésil. Nous avons observé que la Loutre se nourrit principalement de poissons, de crabes et d’écrevisses. Nous n'avons pas observé de saisonnalité à la fois dans l'alimentation ou dans la disponibilité en proies. Cependant, grâce à l’analyse de la variance, nous avons constaté des différences entre la composition du régime et la disponibilité des proies. L’analyse multidimensionnelle montre quant à elle que le régime de la Loutre dans les mangroves est dominé par Brachyura et la disponibilité des proies par: Brachyura, Caridea, Ariidae, Mugilidae, Gerreidae, Centropomidae et Cichlidae, alors que dans le tronçon fluvial, le régime est dominé par : Cichlidae, Caridea et Heptapteridae, et la disponibilité des proies par Characidae, Erythrinidae et Heptapteridae. Selon l'indice de prédation « Ivlev », peu de proies sont consommées le long de la rivière en compraison de leur abondance, la majorité étant sélectionnées. Les loutres préfèrent les proies lentes, peu importe leur taille. Nous avons observé des variations dans le niveau de préférence de la proie entre tronçons, accompagné d’une flexibilité dans le régime. Les loutres ne mangent que peu de proies comparé à leur abondance mais elles montrent une spécialisation de leur comportement alimentaire en mangeant les proies les plus lentes du tronçon.
Revenez au dessus
Resumen:¿Las Nutrias son Generalistas o Prefieren las Presas Más Grandes y Más Lentas? Flexibilidad Alimentaria de la Nutria Neotropical Lontra longicaudis en el Bosque Atlántico
Aunque hay varios estudios que se enfocan en los hábitos alimentarios de Lontra longicaudis, pocos estudios se dirigieron a evaluar su selectividad de presas, y ninguno consideró la movilidad de las presas. En este estudio, informamos acerca de su flexibilidad alimentaria así como su comportamiento alimentario especialista, en dos porciones del Río Mambucaba, en el Sudeste de Brasil. Observamos que se alimentan principalmente de peces, cangrejos y langostinos. No observamos estacionalidad ni en la dieta ni en la disponibilidad en la comunidad de presas. Sin embargo, usando ANOVA, encontramos diferencias entre las dos porciones, en cuanto a la composición dietaria y la disponibilidad de presas. La ordenación Monotónica Multidimensional mostró que la dieta de la nutria en los manglares estuvo dominada por Brachyura, y la disponibilidad de presas por Brachyura, Caridea, Ariidae, Mugilidae, Gerreidae, Centropomidae y Cichlidae, en tanto que la dieta en el tramo de río estuvo dominada por Cichlidae, Caridea y Heptapteridae, y la disponibilidad de presas por Characidae, Erythrinidae y Heptapteridae. De acuerdo al Indice de Electividad de Ivlev, a lo largo del río pocas presas fueron consumidas de acuerdo a su abundancia, siendo la mayoría seleccionadas. Las nutrias prefirieron las presas más lentas, independientemente del tamaño. Observamos variación en el nivel de preferencia hacia la misma presa, en distintos tramos, con flexibilidad en la dieta de la nutria. Las nutrias comieron pocas presas de acuerdo a su abundancia, mostrando en cambio un comportamiento alimentario especialista, comiendo las presas más lentas del tramo.
Vuelva a la tapa
Resumo: As Lontras são Generalistas ou preferem as Presas Maiores e Mais Lentas? Flexibilidade Alimentar da Lontra Neotropical Lontra longicaudis na Mata Atlantica
Apesar da existência de vários estudos anteriores enfocando o hábito alimentar de Lontra longicaudis, poucos estudos procuraram avaliar sua seletividade quanto as presas e nenhum deles considerou a mobilidade delas. Neste estudo, relatamos tanto a flexibilidade como o seu comportamento especialista do seu hábito alimentar em duas partes do rio Mambucaba, Sudeste do Brasil. Observou-se que elas se alimentavam principalmente de peixes, caranguejos e pitús. Não observamos sazonalidade nem na dieta nem na disponibilidade de presas. No entanto, a partir da utilização da análise de Variância, foram observadas diferenças entre os trechos tanto na composição da dieta como na disponibilidade de presas. A técnica de ordenação de escala monotônica Multi-Dimensional mostrou que a dieta da lontra no trecho de manguezal foi dominada por Brachyura e a disponibilidade de presas por Brachyura, Caridea, Ariidae, Mugilidae, Gerreidae, Centropomidae e Cichlidae, enquanto a dieta no trecho do rio foi dominada por Cichlidae, Caridea e Heptapteridae, ea disponibilidade de presas por Characidae, Erythrinidae e Heptapteridae. De acordo com o índice de eletividade de Ivlev, ao longo do rio poucas presas foram consumidas de acordo com a sua abundância, a maioria sendo selecionada. Lontras preferiram as presas mais lenta, não importando o seu tamanho. Observou-se a variação no nível de preferência da mesma presa em trechos diferentes, com flexibilidade na dieta das lontras. As lontras comeram poucas presas de acordo com a sua abundância, mas mostrou comportamento alimentar especialista, comendo a presa mais lenta do local.
Voltar ao topo