|
IUCN/SCC Otter Specialist Group Bulletin
©IUCN/SCC Otter Specialist Group
Volume 13 Issue 1 Pages 1 - 55 (October 1996)
Citation: Miller, I. and Gutleb, A.C.. (1996). Serum Albumin of the Otter (Lutra Lutra L., 1758): An Electrophoretic Study. IUCN
Otter Spec. Group Bull. 13 (1): 14 – 19
Previous
| Contents
|
Next
Serum Albumin of the Otter (Lutra Lutra L., 1758): An Electrophoretic Study
Ingrid Miller1
and Arno Gutleb1
1Institute of Medical Chemistry, University of Veterinary Medicine Josef Baumann-Gasse 1, A-1210 Vienna, Austria
|
| (Received March 19th, 1996, accepted April 12th, 1996) |
|
Abstract: Electrophoretic techniques were used to characterize otter serum albumin in respect to isoelectric point, molecular mass and mobility in the electric field. Comparison with the homologous protein of other carnivores shows great similarities between the investigated members of this zoological class and marked differences to most other mammals.
|
| Keywords: otter, carnivores, serum albumin, electrophoresis |
|
Abbreviations: PAGE = polyacrylamide gelelectrophoresis; pI = isoelectric point; SDS = sodium dodecylsulfate |
INTRODUCTION
During the last years the otter (Lutra lutra) and its environment have become one of the major objects of our research (
Gutleb et al., 1993
;
Gutleb, 1994
). There was little information on otter proteins, comprising only some electrophoretic data in regard to polymorphisms (
Guenther et al., 1981
;
Scheil and Guenther, 1985
) and acute phase protein levels (
Duffy et al., 1994
). Therefore, we started a study on a larger number of otter serum/blood samples (mainly collected from animals found dead) in order to improve knowledge on otter serum proteins, to establish an electrophoretic map of the "normal" serum protein pattern and to determine the effects of sample history (
Miller et al., 1995b
).
Another subject of our interest has been the study of different serum albumins and the comparison of their properties (
Miller and Gemeiner, 1993
). 12 species have been investigated, whereof cat and dog albumins gave markedly different patterns. Thus, comparison with albumin of the otter, belonging to the same zoological class, seemed of particular interest.
MATERIAL AND METHODS
Samples
Otter samples were either collected as sera from healthy individuals or as serum/blood samples from freshly found dead animals (mainly after traffic accidents), of Danish, Hungarian, and Austrian origin. For comparison, serum samples from healthy cats and dogs as well as commercially available human serum albumin (Behring) were used.
Electrophoretic Methods
The following physicochemical characteristics of otter serum albumin were determined electrophoretically:
- The mobility, using routine electrophoresis on cellulose acetate membranes or native polyacrylamide gel electrophoresis (
PAGE
).
- The isoelectric point, i.e. the pH where the overall net charge of the protein is zero, by performing isoelectric focusing in polyacrylamide gels. Depending on the presence/absence of urea and reducing agents, the protein is either in its folded or in its unfolded status; accordingly, the isoelectric point for either the native or the denatured protein may be evaluated.
- The molecular mass, determined by PAGE in the presence of sodium dodecyl sulfate (SDS).
Methods have been described in detail in
Miller and Gemeiner (1993)
.
RESULTS AND DISCUSSION
Albumin is the major plasma protein, important as a transport molecule (for fatty acids, bilirubin, hormones, drugs, ions, etc.), in colloid osmotic regulation, and as easily accessible protein reserve. There are only few general investigations on animal serum albumins, although major disorders are known in humans (
Andersson, 1979
). In a previous study we have investigated the electrophoretic properties of albumins of 12 different species (human, horse, cow, pig, goat, sheep, cat, dog, rabbit, mouse, rat, chicken;
Miller and Gemeiner, 1993
). Especially dog and cat albumin showed markedly different behaviour: they had a higher mobility in the electric field, a higher molecular mass and a more acidic isoelectric point. Preliminary findings suggested similar properties for otters. The results of further, more detailed experiments are summarized in
table 1
, comparing the data also with human serum albumin (the species which is characterized best):
- In routine electrophoresis, otter albumin is faster than most of the other albumins, including dog, but markedly slower than cat. Column 2 of
table 1
gives mobilities in the cellulose acetate membrane system; the trend in native
PAGE
is similar, but not so pronounced. For easier comparison, the mobility of human serum albumin has been set to 1.00 and those of the other species recalculated on this basis.
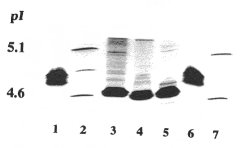
- Isoelectric point: Due to its microheterogeneity, albumin focuses not at a single pH, but produces a series of bands in a limited pH-range. This range is much more acidic for cat and dog than for human, otter albumin can hardly be distinguished from cat albumin.
Fig. 1
shows patterns determined under native conditions. Under denaturing conditions, isoelectric points of albumins are usually higher: the three carnivores give similar pH-ranges of 5.0-5.2, whereas pH 5.4-5.8 was determined for human serum albumin.
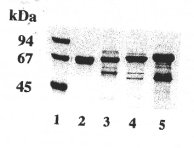
- The molecular masses of the three carnivore albumins are higher than those of all other species we have already investigated. Otter albumin turned out to be the largest molecule of the three (
fig. 2a
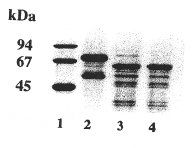
). An interesting fact was noticed when investigating some degraded blood samples from dead animals which had not been found/collected immediately. These specimens showed a markedly smaller albumin (only 62 kDa, see
fig. 2b
), most likely due to cleavage. Similar effects could be generated in vitro by limited enzymatic digestion of the intact "normal" molecule (e.g. with trypsin).
On the basis of these findings we were interested to test also samples from other carnivores, as we supposed these albumin properties to be "carnivore-specific". Specimens could be obtained from mink (Mustela vison), fox (Vulpes vulpes), wolf (Canis lupus), and polar bear (Ursus maritimus). Indeed, wolf samples were very similar to the dog. Also mink, fox, and polar bear showed patterns comparable to the already investigated carnivores, but with properties "intermediate" between cat and dog (data not shown).
|
Table 1: Frequency of occurrence of the prey items found in the spraints of the Neotropical otter (Lontra longicaudis) in the Mambucaba river in each stretch by season. |
| Species |
Mobility
†
|
Molecular Mass
[kDa] |
Isoelectric point
(native molecule) |
|
| human |
1.00 |
66 |
4.7-4.95 |
|
otter |
1.08 |
73 |
4.6-4.7 |
|
cat |
1.13 |
70 |
4.6-4.7 |
|
dog |
1.04 |
71 |
4.5-4.65 |
|
|
† mobility on cellulose acetate membranes |
|
Figure 1: Isoelectric focusing without denaturing additives; T = 3.5 %, C = 2.7 % (piperazine diacrylamide as crosslinker), Coomassie staining. Standard (lanes 2 and 7): equal amounts of amyloglucosidase (pI 3.6), trypsin inhibitor (pI 4.6), ß-lactoglobulin A (pI 5.1) and carbonic anhydrase II (pI 5.9). Human serum albumin in lanes 1 and 6; serum samples: otter (3), dog (4), and cat (5).
Click for larger version |
|
|
| Figure 2a |
Figure 2b |
| SDS-PAGE in a gradient gel with 10 - 15 % T, 2.6 % C (stacking gel 5 % T, 2.6 % C), Coomassie staining. Standard (lane 1): LMW (Pharmacia), containing: phosphorylase B (94 kDa), bovine serum albumin (67 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and *-lactalbumin (14.4 kDa). |
|
Samples: Click images for larger versions
2
a) human serum albumin (2); cat (3), dog (4), otter (5) serum
2 b) three different otter sera: in lane 2 normal pattern, in lanes 3 and 4 older otter samples which show degraded albumins.
|
Until now there is no substantial explanation for this different behaviour of the carnivore albumins. Purification, fingerprinting (i.e. comparison among proteolytic or chemical digests) and/or sequence analysis would be necessary to get information on the primary structure of the respective protein. There are no data on protein fragmentation and no complete sequence data yet, not even on cat and dog albumin. Data on total protein hydrolysis of cat albumin show that it contains more acidic amino acids than human serum albumin (
Dandeu et al., 1991
). Similarly, protein structure and folding is only known for the human homologue (
He and Carter, 1992
). More information on albumin properties would be valuable also for zoologists, as this protein has been suggested to serve as an evolutionary clock and as a marker for the relationship of species (
Sarich, 1969
).
CONCLUSIONS
Otter serum albumin shows properties similar to both, dog and cat albumin. It has the same isoelectric point range as cat serum albumin, but it differs slightly in molecular mass and mobility from the feline and canine homologue. All carnivore albumins investigated showed properties quite similar to each other and markedly different to most of the other species. Thus, electrophoresis can be applied as a useful tool for species identification/differentiation, to monitor the protection of endangered species and to detect offences against it (as already shown in
Miller et al., 1995a
). Further investigation on protein structure should be undertaken to reveal the molecular basis for the differences noticed in electrophoretic behaviour.
Acknowledgements - The otter samples were kindly supplied by Dr. Aksel B. Madsen (National Environmental Research Institute, Denmark), Dr. Pim Leonards (Institute for Environmental Studies, The Netherlands), Dr. Gabor Nechay (Ministry for Environment and Regional Policy, Hungary), and Dr. Andreas Kranz (Institute for Wildlife Biology and Game Management, University for Agriculture, Austria). We are also very grateful to Dr. Helmut Pechlaner (Zoo Schoenbrunn, Austria) for serum samples from polar bear and wolf, and to Dr. Cathrine Foyn Brunn (Institute of Clinical Medicine, University of Tromsø, Norway) for mink and fox specimens (fractions from chromatographic separations).
REFERENCES
Andersson, L.-O. (1979). Serum albumin. In: Blombaeck, B., Hanson, L.A., Winberg, H. (Eds.), Plasma Proteins, John Wiley & Sons, Chichester, pp. 43-54
Dandeu, J.P., Rabillon, J., Guillaume, J.L., Camoin, L., Lux, M., David, B. (1991). Isolation and purification of cat albumin from cat serum by copper ion affinity chromatography: further analysis of its primary structure. J. Chromatogr., 529: 475-484
Duffy, L.K., Bowyer, R.T., Testa, J.W., Faro, J.B. (1994). Chronic effects of the Exxon Valdez oil spill on blood and enzyme chemistry of river otters. Environment. Toxicol. Chem., 13: 643-647
Guenther, A., Scheil, H.-G., Reuther, C. (1981). Serumproteine und Erythocyztenenzyme beim Fischotter (Lutra lutra L.: Carnivora). Zool. Anz. Jena, 207: 123-129
Gutleb, A.C., Schenck, C., Staib, E. (1993). Total mercury and methylmercury levels in fish from Peru. IUCN Otter Spec. Group Bull., 8, 16-18
Gutleb, A.C. (1994). Heavy metals, organochlorpesticides and PCBs in spraints of the otter (Lutra lutra) from North-Eastern Slovenia. IUCN Otter Spec. Group Bull., 10: 31-34
He, X.M., Carter, D.C. (1992). Atomic structure and chemistry of human serum albumin. Nature, 358: 209-215
Miller, I., Gemeiner, M. (1993). Peculiarities in electrophoretic behaviour of different albumins. Electrophoresis
14: 1312-1317
Miller, I., Gutleb, A.C., Kranz, A., Gemeiner, M. (1995a). Forensics on wild animals: Differentiation between otter and pheasant blood by electrophoretic methods. Electrophoresis
16: 865-868
Miller, I., Gutleb, A.C., Gemeiner, M. (1995b). Two-dimensional electrophoresis for the study of blood/serum proteins of the otter, a protected species. Electrophoresis
16: 1193-1198
Sarich, V.M. (1969). Pinniped origins and the rate of evolution of carnivore albumins. Syst. Zool., 18: 286-295
Scheil, H.-G., Guenther, A. (1985). Elektrophoretischer Vergleich einiger Erythocytenenzyme der Otter Lutra lutra (L., 1758), Lutra canadensis (Schreber, 1776) und Pteronura brasiliensis (Gmelin, 1788). Saeugetierkundliche Mitteilungen
32: 63-66
Previous
|
Contents
|
Next
|



