IUCN/SSC Otter Specialist Group Bulletin

|
©IUCN/SCC Otter Specialist Group Volume 28 Issue 2 Pages 62 - 118 (October 2011) Citation: Cousins, L., Tansley, D. and Hepburn, L. (2011). Investigation into the Dietary Habits of the Eurasian Otter (Lutra lutra) in the County of Essex . IUCN Otter Spec. Group Bull. 28 (2): 76 - 83 Investigation into the Dietary Habits of the Eurasian Otter (Lutra lutra) in the County of Essex Leslie Cousins1, D. Tansley2 and L. Hepburn1
1Department of Biological Sciences, University of Essex, Wivenhoe Park, Colchester.CO4 3SQ. e-mail: lcousi@essex.ac.uk |


 |
| Received 12th July 2011, accepted 29th October 2011 |
| Abstract: Monitoring throughout the county of Essex has shown annual widening of otter distribution. There is, however, room for expansion and some areas remain un-colonised. This paper reports a snapshot study of spraints collected from within the areas of known distribution, providing additional insight on a growing population. Prey remains were identified to family level and data used to calculate trophic breadths over the range of stream orders. Investigative comparisons were used to detect changes in diet with stream order. Further consideration was given to the importance of crayfish predation (e.g. the signal crayfish Pacifastacus leniusculus).Within the sample (n= 54) from four stream orders (Strahler 2-5), fish occurred most frequently (67%). Other groups included; invertebrates 20%, birds 7% and mammals 6%. Crayfish comprised 4% of the sample. There were no significant differences between Trophic Niche Breadth and stream order (H*=2.73, P>0.05), a finding strengthened by subsequent statistical analysis of the data. Dietary composition was consistent within the range and period studied. Extended research could determine seasonal variation and the extent to which available prey assemblage limits distribution against wider environmental and biological variables. . |
| Keywords: distribution, trophic niche breadth, Strahler classification, predation |
| Française | Español |
The Eurasian otter (Lutra lutra) population in the county of Essex and East Anglia is experiencing growth and becoming re-established after regional extinction (Mason and MacDonald 2003).
Historically, the otter was common throughout Britain (Stephens 1957). However, by the early 1960’s hunt returns where showing a sharp reduction in numbers (Chanin and Jefferies 1978).In response to growing conservation concern a series of national surveys were instigated (Hewer 1974, Lenton et al., 1980).The second national survey reported an absence of otter signs in Essex (Strachan et al., 1990). The decline and eventual disappearance of otters was attributed to the effects of persistent toxic pollutants compounded by habitat destruction and direct persecution (Macdonald and Mason 1983; Mason and Macdonald, 1986; Mason, 1989; Strachan and Jefferies, 1996; Jefferies and Hanson, 2000). Environmental pollutants identified as harmful to otters included; organochlorine pesticides; heavy metals and polychlorinated biphenyls (PCBs).
Re-colonisation was facilitated by the efforts of multiple agencies combined within the Joint Otter Group and other agencies working to reverse environmental degradation. Habitat restoration and targeted re-introductions successfully re-established a small yet viable population (Jefferies et al.,1986, 2000). A survey in 1991 (Strachan and Jefferies, 1996), identified the presence of field signs indicating otter usage. Successive surveys have consistently shown increases in the extent to which the species uses local water courses (Tansley, 2008, 2009, 2011). Annual monitoring of the Essex population is tracking progress, which is essential, as the population has yet to reach carrying capacity and still has potential to expand (Crawford, 2010). This study aims to complement survey distribution data with additional information, through dietary analysis, on how a growing otter population uses available resources.
AIM AND OBJECTIVES
Aim
To determine the diet and feeding habits of L. lutra across the county of Essex
Specific Objectives
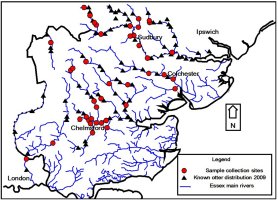
- To produce a map communicating the distribution of collected samples in relation to the known distribution of this species.
- To identify all prey species to family level.
- To determine the relative contributions of each family to otter diet in relation to distribution.
- To compare trophic breadth indices to identify geographical patterns in relation to stream order.
- To determine the importance of invasive crayfish species to otter diet.
Essex is a low-lying county in eastern England. The largest of the counties rivers and tributaries included in this study are; the Stour, Colne, Chelmer and Roding. Spraint samples (n=54) were collected during the spring and summer of 2010 from field locations known to have been previously used by otters (Figure 1, Tansley, 2009). Samples were wrapped in aluminium foil, sealed in plastic sample bags, tagged and stored frozen.
Stream order was allocated to each sample using the system described by Strahler (1952). Streams originating from source were allocated the first order. In this system an nth order stream always flows downstream from the confluence of two (n-1)th order streams.
Samples were oven dried at 60 °C for 12-24 hours until completely desiccated, then carefully crumbled by hand to separate undigested prey remains. Once separated identification of prey remains to family level was aided by published keys (Conroy et al., 2005, Teerink 1991, Day 1966). Binocular Leica Zoom 2000 and Nikon 104 microscopes were used for analysis. The bulk percentage dry weight of prey items was estimated by eye (Wise et al., 1981). Data gathered were used to calculate the percentage (%) frequency occurrence of prey groups (P=number of spraints containing X / total number of spraints x 100).
The relative (R) percentage (%) frequency of occurrence for each prey group(R = occurrence of X / number of groups x 100). Trophic niche breadth (TNB) for each observation using TNB=1/RΣpi2 Levins’ index (Feinsinger et al., 1981). Where pi is the estimated proportion of prey type within each sample and R is the total number of prey types observed. Using descriptive statistics, homoscedasity was assessed for each group before and after various transformations. Despite transformation, neither normality nor homoscedasity were achieved. Therefore, analysis of data required the use of the Kruskal-Wallis (H) test between trophic niche breadth (TNB) and stream order. Additionally, the Spearman’s test was used to look for correlation between ranked TNB and stream order.
A contingency table was constructed to calculate expected values enabling a chi squared (Χ2) test of association between stream order and prey groups. Arcsine transformed frequencies of prey families were compared between stream orders using the paired t-test. The statistical analyses of data were performed using SPSS software.
RESULTSSpraints (n=54) were collected from rivers of the orders; two to five (Figure 1). Six of the samples (11%) came from second order streams. Third order streams contributed the highest proportion of 20 samples (37%). Seventeen samples were taken from streams of the fourth order (31%). The remaining eleven samples (20%) where collected from fifth order streams. The dry weight of spraints ranged from 0.34g to 7.91g, mean 2.046g (SE 0.195).
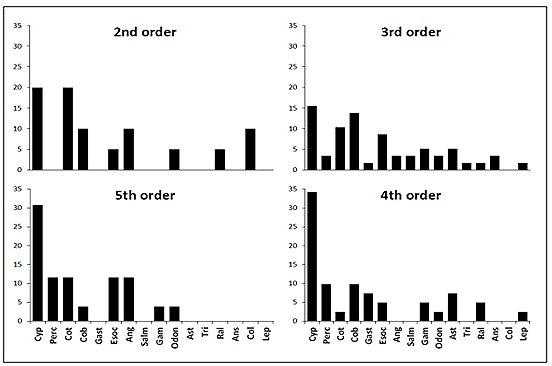
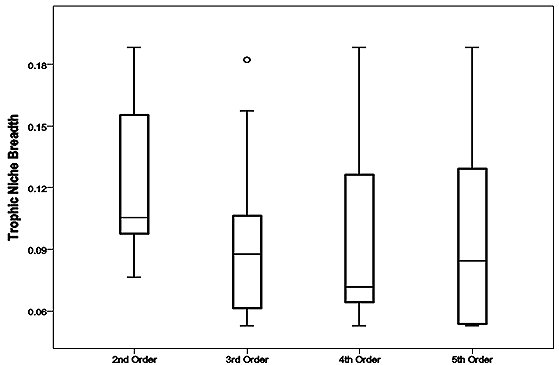
Otters predated animals from four groups comprised of eight families of fish, four families of invertebrate, three families of bird and one family of mammal (Table 1). Percent frequency occurrence values of prey families within each stream order (Figure 2) provided a description of predation within each stream order, these values were used to calculate related trophic niche breadths (Figure 3). The distribution of TNB values within each group was skewed. Second order streams had a median value of 0.1. The median TNB value of third order streams was 0.09. In the group of fourth order streams, the distribution was highly skewed and had a median of 0.07. Within the fifth order group of samples the TNB median was 0.08.
Comparison of these data could not separate populations, there being no significant difference between trophic niche breadths and stream order, H* =2.73, P>0.05. There was no correlation between ranked niche breadth data and stream order (rS = -0.116, P>0.05). Prey occurrence data was tested for association with stream orders and none were found, e.g. the number of occurrences of Cyprinidae fish families within spraints were not significantly different between either of the stream orders investigated, χ23= 5.41, P<0.05.
A comparison of the, arcsine transformed, proportion of each prey type found no significant differences (P>0.05) in distribution among stream orders (Table 2).
The frequency occurrence of prey within the diet of the Essex population was congruent with previous studies of eutrophic systems (e.g. Weir and Bannister, 1973, 1977; Jarman, 1979; Woodroffe, 1994), and consistent with evolutionary adaptation. In this study fish were the most frequently taken food items, 67%. In terms of volume, fish may contribute a larger proportion of diet than has been measured here. Due to problems associated with bulk estimations (Carss and Nelson, 1998) no attempt had been made to estimate the volume of fish eaten, whole or part.
Insects and crustaceans featured regularly in the diet (19.9%). Consistent occurrence across stream orders suggests invertebrates are an important dietary component to this population. Carss and Parkinson (1996) have described how well fed, captive otters actively pursue and consume aquatic insects. The benefit and importance of invertebrates to the diet of otters is considered by Taylor et al. (2010).
Otters are considered beneficial as a source of biological control of invasive crayfish species (Reeve, 2004). In this study crayfish occurred in 4% of the sample. Crayfish predation is identified by the presence of carapace or other cuticle fragments within spraints. This method is limited by providing only presence or likely absence of occurrence and not volumetric data. To fully understand habits, a longer study would be needed to identify spatial and seasonal variations in crayfish predation.
Birds and mammals were an infrequent, though regular, feature in the diet of the Essex population (bird 7.4%, mammal 5.5%). The families predated (e.g. Ralliform, Anserform and Leporidae) were those otters are most likely to encounter, and the infrequency suggests predation is opportunistic.
Amphibians area group of potential prey known to feature within the diet of otters (e.g. Clavero et al., 2003). The absence of amphibian prey from the sample could be due to the timing and habitat focus of sample collection. Observations have shown that otters feed on amphibians during the spawning season in early spring (Mason and Macdonald, 1986; Weber, 1990). This study focused on river habitats, excluding standing waters that are preferred breeding sites for frogs, toads and newts (Baker et al., 2011).
Comparison of TNB and one physical attribute of habitat variability (stream order) found no significant change in diet between rivers in terms of size and discharge. This study has detailed dietary norms of the local population. As range is a function of habitat quality (Jefferies and Woodroffe, 2008), these data could provide a baseline with which to gauge the prey assemblage of water courses yet to support otters.
An extended comparison of distribution, diet and broader habitat quality (eg. the standardised River Habitats Survey, Raven et al., 1997, 1998; Fox et al., 1998) of used and un-colonised rivers could provide informative results. Indices such as the RHS collect a range of physical and biological habitat attributes, not included in this study, which may have influence over the ecology and distribution of otters.
Acknowledgements - I would like to thank the following people for all their generous help; Russell Smart for laboratory assistance, Odette Robson for help and suggestions. Stephen Wilkinson, Sue Manning, Patricia Clegg and Peter Margetts for help collecting spraint samples.
REFERENCES
Baker, J., Beebee T., Buckley, J., Gent, T. and Orchard, D. (2011). Amphibian Habitat Management Handbook. Amphibian and Reptile Conservation, Bournemouth.
Carss, D.N. and Nelson, K.C. (1998). Cyprinid prey remains in otter Lutra lutra faeces: some words of caution, J. Zool, 245: 238- 244
Carss, D.N. and Parkinson, S.G. (1996). Errors associated with otter Lutra lutra faecal analysis. I. Assessing general diet from spraints. J Zool, 238: 301- 317
Chanin, P.R.F. and Jefferies, D.J. (1978). The decline of the otter Lutra lutra L. in Britain: an analysis of hunting records and discussion of causes. Biol J Linn Soc. 10: 305-328
Clavero, M., Prenda, J., Delibes, M. (2003). Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J. Biogeogr, 30: 761-769.
Conroy, J.W.H., Watt, J., Webb, J.B., Jones, A. (2005). A guide to the identification of prey remains in otter spraint, 3rd edition. London. The Mammal Society.
Crawford, A. (2010). Fifth otter survey of England. 2009-2010. Technical report. Bristol, Environment Agency.
Day, M.G. (1966). Identification of hair and feather remains in the gut and faeces of stoats and weasels. J Zool, London, 148: 201-217.
Feinsinger, P., Spears, E.E., Poole, R.W. (1981). A Simple Measure of Niche Breadth, Ecology, 62: 27-32
Fox, P. J., Naura, M., Scarlett, P. (1998). An account of the derivation and testing of a standard field method, River Habitat Survey. Aquat Conserv, 8: 455-475.
Hewer, H.R. (1974). The Otter in Britain-a Second Report. Oryx, 12: 429-435.
Jarman, R. (1979). Otter survey of the Somerset Levels 1977-78, Bridgewater, The Somerset Trust for Nature Conservation
Jefferies, D. J., Wayre, P., Jessop, R. M., Mitchell-Jones, A. J. (1986). Reinforcing the native otter Lutra lutra population in East Anglia: an analysis of the behaviour and range development of the first release group. Mammal Rev, 16: 65-79.
Jefferies, D.J. Wayre, P., Shuter, R. (2000). A brief history of the Otter Trust’s successful programme of repopulating lowland England with otters bred in captivity with a special emphasis on East Anglia. Otters, Journal of the Otter Trust, 3(4): 105-117.
Jefferies. D.J, Hanson, H.M. (2000). The role of dieldrin in the decline of the otter (Lutra lutra) in Britain: the analytical data. In: Conroy, J.W.H., Yoxon, P., Gutleb, A.C. (eds.), Proceedings of the First Otter Toxicology Conference, Skye, Sept 2000 (95-143): International Otter Survival Fund, Broadford, Scotland.
Jefferies, D.J., Woodroffe, G.L. (2008). Carnivores, Mammals of the British Isles: Handbook 4th Edition, In: Harris, S., Yalden, D.W. (eds.) 437- 447, Southampton, The Mammal Society
Lenton, E.J., Chanin, P.R.F., Jefferies, D.J. (1980). Otter survey of England 1977-79. Nature Conservancy Council, London.
MacDonald, S.M., Mason, C.F. (1983). Some factors affecting the distribution of Lutra lutra. Mammal Review, 13:1-11
Mason, C.F., MacDonald S.M. (1986). Otters, Ecology and Conservation. Cambridge. Cambridge University Press.
Mason, C.F. (1989). Water Pollution and Otter Distribution: A Review, Lutra, 32: 97- 131
Mason, C.F., Macdonald S.M (2003). The otter Lutra lutra in Essex 1996- 2002. Essex Naturalist, 20 (New Series): 159- 176.
Raven, P.J., Fox P., Everard M., Holmes N.T.H., Dawson F.H. (1997). River Habitat Survey: a new system for classifying rivers according to their habitat quality. In: Boon P.J., Howell D.L. (eds): Freshwater Quality: 215–234. Defining the Indefinable?, The Stationery Office, Edinburgh.
Raven, P. J., Holmes N.T.H., Dawson F.H., Everard, M. (1998). Quality assessment using River Habitat Survey data. Aquat. Conserv.: Marine and Freshwater Ecosystem 8: 477–499.
Reeve, I.D. (2004). The removal of the North American signal crayfish (Pacifastacus leniusculus) from the River Clyde. Scottish Natural Heritage Commissioned Report No. 020.
Stephens, M.N. (1957). The Otter Report. University Federation for Animal Welfare, Potters Bar.
Strachan, R., Birks, J.D.S., Chanin, P.R.F., Jefferies, D.J. (1990). Otter survey of England 1984-1986. Nature Conservancy Council, Peterborough.
Strachan, R., Jefferies, D.J. (1996). Otter survey of England 1991-1994. Vincent Wildlife Trust, London.
Strahler, A.N. (1952). Dynamic Basis for Geomorphism, Geol Soc Am Bull, 63: 923-938.
Tansley, D. (2008). Essex Otter Survey 2007. The Essex Wildlife Trust/ Water for Wildlife.
Tansley, D. (2009). Essex Otter Survey 2008. The Essex Wildlife Trust/ Water for Wildlife.
Tansley, D. (2011). Essex Otter Survey 2009/10. The Essex Wildlife Trust/ Water for Wildlife.
Taylor, R.E. Forman. D.W. Greig. C., Parry, G.S. (2010). Otters, the unexpected entomophage? Biologist, 57: 122-126.
Teerink, B.J. (1991). Hair of West-European mammals, Atlas and identification key. Cambridge, Cambridge University Press.
Weber, J.M. (1990). Seasonal exploitation of amphibians by otters (Lutra lutra) in north-east Scotland. J. Zool, 220: 641-651.
Weir, V., Bannister, K.E. (1973). The food of the otter in the Blakeney area. Norfolk Naturalists Society, 22: 377-382.
Weir, V., Bannister, K.E. (1977). Additional notes on the food of the otter in the Blakeney area, Norfolk Naturalists Society, 24: 85-88
Wise, M.H., Linn, I.J., Kennedy, C.R. (1981). A comparison of the feeding biology of mink Mustela vison and otter Lutra lutra. J Zool, London, 195: 181-213.
Woodroffe, G.L. (1994). The status and distribution of otter (Lutra lutra L.) in North Yorkshire, Naturalist, 119: 23-25.
Résumé : Enquête sur les Habitudes Alimentaires de la Loutre d’Europe (Lutra lutra) dans le Comté d'Essex
Le suivi de la Loutre dans le Comté d'Essex a montré un élargissement annuel de sa répartition. Il existe effectivement des possibilités d'expansion mais certaines zones demeurent non colonisées. Cet article rapporte l’analyse rapide d’épreintes recueillies sur les zones de répartition connue, apportant des éléments supplémentaires sur une population croissante. Les restes de proies ont été identifiés jusque la famille et les données obtenues ont permis de calculer des valeurs trophiques sur l’ensemble des tronçons du continuum fluvial. Diverses enquêtes ont été comparées afin de détecter des variations alimentaires en fonction de la situation sur le continuum fluvial (classe de courant). Un examen plus approfondi a mis l’accent sur l'importance de la prédation des écrevisses (Pacifastacus leniusculus). Au sein de l'échantillon (n=54) regroupant quatre classes de courant (Strahler 2-5), les poissons sont les plus fréquents (67%). D'autres groupes sont aussi présents; les invertébrés 20%, les oiseaux 7% et les mammifères 6%. Les écrevisses composent 4% de l'échantillon. Il n'y avait pas de différences significatives entre l’ampleur de la niche trophique et la classe de courant (H * = 2,73, P>0,05). La composition alimentaire est restée stable durant l’étude et sur l’ensemble de la zone suivie. Des recherches plus poussées permettraient d’apprécier des variations saisonnières et évaluer dans quelle mesure la disponibilité des proies limite l’expansion de l’espèce en parallèle de variables environnementales et biologiques.
Revenez au dessus
Resumen: Estudio de los Hábitos Dietarios de la Nutria Euroasiática (Lutra lutra) en el Condado de Essex
Un monitoreo que abarca el condado de Essex ha mostrado una ampliación de la distribución de la nutria. Sin embargo, espacio para expansión y algunas áreas se mantienen sin colonizar. Este artículo es resultado de un estudio acotado de fecas colectadas dentro de las áreas de distribución conocida y aporta información adicional sobre una población en crecimiento. Los restos de presas fueron identificadas al nivel de familia y los datos usados para calcular la amplitud trófica en todo el rango de órdenes de los cursos fluviales. Se usó la comparación de investigaciones para detectar cambios en la dieta según el orden del curso fluvial. Se le dio otra importancia a la predación de cangrejos (por ej. el cangrejo señal Pacifastacus leniusculus). De la muestra (n=54) de cuatro órdenes de cursos fluviales (Strahler 2-5), los peces fueron más frecuentes (67%). Otros grupos presentes fueron: invertebrados 20%, aves 7% y mamíferos 6%. Los cangrejos comprendieron el 4% de la muestra. No se hallaron diferencias significativas entre las Amplitudes de Nicho Trófico según el orden del curso fluvial (H* =2.73, P>0.05), este resultado está reforzado por posteriores análisis estadísticos de los datos. La composición dietaria fue estable dentro del área y período estudiados. Un estudio más amplio podría determinar variaciones estacionales y la medida en que el ensamble de presas disponibles limita la distribución frente a las más amplias variables ambientales y biológicas.
Vuelva a la tapa