IUCN/SSC Otter Specialist Group Bulletin

|
©IUCN/SCC Otter Specialist Group Volume 28 Issue 2 Pages 62 - 118 (October 2011) Citation: Hussain, S.A., Gupta, S.K. and de Silva, P.K. (2011). Biology and Ecology of Asian Small-Clawed Otter Aonyx cinereus (Illiger, 1815): A Review . IUCN Otter Spec. Group Bull. 28 (2): 63 - 75 Biology and Ecology of Asian Small-Clawed Otter Aonyx cinereus (Illiger, 1815): A Review Syed Ainul Hussain1, Sandeep Kumar Gupta1 and Padma Kumari de Silva2
1Wildlife Institute of India, Post Box # 18, Dehra Dun, 248 001, Uttarakhand, India . e-mail: hussain@wii.gov.in |
| Abstract: The Asian small-clawed otter is the smallest among the 13 extant species of otters. It has a large distribution range extending from India in South Asia through Southeast Asia up to Taiwan and Philippines in the east and Southern China in the north. It is considered ‘Vulnerable’ due to habitat loss and degradation, depletion of prey species and exploitation. Being adapted to live in shallow streams and water bodies, they are more vulnerable to modification of these habitats by anthropogenic as well as climate change impacts. This paper summarizes the state of knowledge on the biology and ecology of this little known species. Over the years, the IUCN SSC Otter Specialist Group has developed a cadre of biologist across Asia to conduct field surveys and has popularized otter conservation by promoting otter as the ambassador of wetlands. However, concerted effort is needed for its long-term survival. Policy based action, research on factors affecting survival, habitat-based actions on creation and where required expansion of protected areas and communication and awareness building among local communities are suggested. |
| Keywords: Asian small-clawed otter, species range, biology, ecology, habitat, genetics, conservation |
| Française | Español |
Otters belong to the mammalian order Carnivora and family Mustelidae. They are adapted for a semi-aquatic life with well-developed webs and a tapering tail, which helps in propulsion. Of the 13 extant species of otters distributed worldwide, five species; Eurasian otter (Lutra lutra), Smooth-coated otter (Lutrogale perspicillata), Hairy-nosed otter(Lutra sumatrana), Asian small-clawed otter (Aonyx cinereus) and Sea otter (Enhydra lutris) occur in various freshwater, coastal and marine ecosystems of Asia and Asia Pacific regions. The Asian small-clawed otter is the smallest otter in the world (Harris, 1968; Foster-Turley and Santiapillai, 1990) rarely weighing more than 5 kg. This species has unique hand-like front paws with reduced nails, which are well adapted for catching small vertebrate and invertebrate prey in shallow and murky water.
Based on canonical variate and Wagner analysis of morphological data from the 13 extant taxa, van Zyll de Jong (1987; 1991) identified two principal clades – the first group includes Amblonyx, Aonyx, Enhydra and Lontra, while the second group consists of Lutra, Lutrogale and Pteroneura. Phylogenetic relationships based on Willemsen’s (1992) evaluation of morphological characters from extant and extinct otter taxa, identified three principal clades - Lutrini, Aonychini and Enhydrini, with Lontra, Lutra and Lutrogale in the first, Aonyx and Amblonyx in the second, and Enhyra in the third clades, respectively. The phylogenetic analysis of the complete nucleotide sequence of the mitochondrial cytochrome b gene by Koepfli and Wayne (1998) suggested that otters are divided into three primary clades: (i) the north American river otter (Lutra canadensis), neotropical otter (Lutra provocax) and marine otter (Lutra feline) (ii) the sea otter (Enhydra lutris), Eurasian otter (Lutra lutra), spotted necked otter (Lutra maculicolis), cape clawless otter (Aonyx capensis), Asian small-clawed otter and (iii) the giant otter (Pteronura brasiliensis). A recent genetic analysis based on maximum parsimony, maximum likelihood and Bayesian inference showed that hairy nosed otter (Lutra sumatrana) and Eurasian otter (Lutra lutra) are sister taxa, whereas Asian small-clawed otter (Aonyx cinerea) is sister to smooth-coated otter (Lutrogale perspicillata) (Koepfli et al., 2008).
The Asian small clawed otterhas 2n=38 chromosomes. The X chromosome is metacentric, whereas the Y chromosome is acrocentric (van Zyll de Jong, 1987). The cytochrome-b sequences suggest that the Asian small-clawed otter and the Cape clawless otter (Aonyx capensis) are sister taxa with 10.4% sequence divergence and an estimated divergence time of 5 million years ago (Koepfli and Wayne, 1998). Thus, generic separation of Aonyx and Amblonyx may not be warranted. In view of this, the IUCN SSC Otter Specialist Group decided to use its generic name Aonyx in contrast to Amblonyx as proposed by Rafinesque (1832) and Pocock (1941).
Habitat loss and illegal trade for fur caused the rapid decline of the otter populations worldwide. Comparative genetic study conducted for assessment of variation in historical and present population indicates the loss of genetic variation among several species. For example; analysis of microsatellite allelic data revealed that the pre-fur trade (exploited population of 18th and 19th centuries) population of Sea otter (Enhydra lutris) had significantly more variation than all the extant sea otter populations (Larson et al., 2002). Analysis of microsatellite DNA variation in contemporary and historical samples, including the museum specimens covering a time-span from the 1880s to the 1960s provided indications of a recent bottleneck and loss of genetic variability in Eurasian otter (Lutra lutra) (Pertoldi et al., 2001). Mucci et al. (2010) conducted a genetic survey on the biological sample of otter collected from European countries and demonstrated that the genetic variation is low in mitochondrial DNA D-loop region and microsatellite markers.
SPECIES RANGE
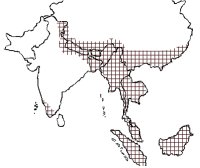
The Asian small-clawed otter has a large distribution range from India in South Asia through Bangladesh, Myanmar, Thailand, and Indonesia in Southeast Asia to Philippines and Taiwan in the east and Southern China in the north. Its presence has been confirmed from India, Nepal, Bhutan, Bangladesh, Myanmar, South China and Hainan Islands, Thailand, Laos, Brunei, Malaysia, Vietnam, Indonesia, Taiwan and Philippines (Foster-Turley and Santiapillai, 1990; Hussain, 1999; Gonzalez, 2010) (Fig. 1). In Vietnam it appears to have a relatively widespread distribution with reports of its occurrence from the northern highlands limestone, Hoang Lien mountain range, northern and central Annamites, central Indochina limestone, Ke Go/Khe Net lowlands, the eastern plains dry forest, lowland Dong Nai watershed and the Mekong delta. It has been found in lower montane evergreen forests, peat swamp forests, freshwater wetlands, freshwater swamp forests and coastal wetlands (Roberton 2007). The records from Vietnam ranged from 50-600 m in elevation, although it has been found at up to 1500 m in other countries (Duckworth, 1997). The Asian small-clawed otter is believed to be extinct in Hong Kong (Foster-Turley and Santiapillai, 1990) and rediscovered in Singapore (Sivasothi, 1996).
 |
|
Figure 1.
Distribution of Asian small-clawed otter in South and Southeast Asia. (click for larger version) |
The Asian small-clawed otters are nocturnal and crepuscular where they live near people in northern Malaysia. At night their chirps can be heard in rice fields. They are occasionally seen in the early morning or around dusk. In recent years, in England it has established itself in the wild after escaping from captivity (Jefferies, 1989, 1991).
In India, the Asian small-clawed otter is reported to occur in north India from the Himalayan foothills of Himachal Pradesh (Kulu), West Bengal, Assam hill ranges as well as in South India, in the higher ranges of the hills in Coorg (Karnataka), Ashambu, Nilgiri and Palani hills (Tamil Nadu) and some places in Kerala (Pocock, 1941; Prater, 1948; Hussain, 1999). However, in recent years its occurrence has been confirmed from Western Ghats and from the northeast India (Hussain, 1999).
Three sub-species were recognized (Harris, 1968); A. c. concolor from the Himalayas from Kulu eastward to Assam, Meghalaya and upper Burma and A. c. nirnai from the Westen Ghats (Pocock, 1941); and A. c. cinereus from Southeast Asia. Corbet and Hill (1992) recognized two subspecies from Southeast Asia Amblonyx (Aonyx) c. fulvus from Lao Key, Tonkin, Vietnam (Pohle, 1920) and Amblonyx (Aonyx) c. wurmbi (Sody, 1933) from near Poeger, Watangan Mountains, on east Java.
MORPHOLOGY
The Asian small-clawed otter has distinctive webbed feet, with the third and fourth digits markedly longer than second and fifth on each foot. All claws are reduced to small rudiments, which do not project beyond the tips of the digits. It is small in size as compared to other otter species, head and body measuring 406-635 mm, tail length 246-304 mm and the total length from snout to the tip of the tail is around 652-939 mm. Its weight ranges between 2.7-5.4 kg (Walker, 1975). The females are on an average, only a little smaller than the males (Harris, 1968).
The Asian small-clawed otter is principally distinguished from the Eurasian and the smooth-coated otter in external characters by the structure of the feet, which are considerably narrower with the digits tied closer together by shallower, narrower and more emarginated webs, which do not extend along the digital pads and are sparsely covered below with short hair. Also the claws, except in small cubs, are minute, erect spikes not projecting beyond the end of the digital pads. The planter pads are better developed, sub-symmetrical and four lobed, the inner main lobe being prolonged backwards by the pollical and hallucal elements. The rhinarium is inverted ‘V’ shaped similar to smooth-coated otter, its anterior surface is directed forwards and posterior margin is straight (Pocock, 1941). In the field, tracks of small-clawed otter can be differentiated from tracks of other otters by smaller size (width, 4.5 cm), absence of claw marks, incomplete webbing between fingers and toes, longer middle digit compared with other digits, and relatively long fingers (Lariviere, 2003).
Skull
The skull is shorter and broader, width of the cranium being over half the total length, and the dorsal profile not so depressed. The bulla is better developed, almost reaching the para-occipital process behind. The post-orbital area is short and only in well-developed skulls has an abrupt constriction a little distance behind the post-orbital process. The dentition is characterized by the absence of upper premolar, there being only four instead of five post-canine teeth above. The last two upper teeth (pm4 and m3) are larger in size than the Smooth-coated and the Eurasian otters.
Colour
The dorsal body colour is typically dark brown, sometimes with a tawny or rufous tinge. Often tips of the contour hairs are paler, but rarely white, giving the grey or ashy tint. The ventral side is generally paler brown than the upper, often showing a grey cast. The edge of the upper lip, chin, cheek, side of the neck and throat are grey or nearly white, sometimes sharply, sometimes comparatively weakly contrasted with the darker upper tint of the head and the neck (Pocock, 1941).
PHYSIOLOGY
Metabolic rates
During surface swimming the Asian small-clawed otter and their larger relatives propel their body using all four limbs, rowing with the forelimbs and paddling with the hind limbs (Fish, 1994). While swimming under water, propulsion is provided via dorso-ventral undulation of body and tail, similar to those of other truly aquatic mammals. Thus, the underwater swimming metabolism of Asian small-clawed otters is higher than that of fully aquatic mammals, but lower than those of paddling semi aquatic mammals (Borgwardt and Culik, 1999).
The metabolic rate of Asian small-clawed otter on land is similar to those of other otter species and about double than those of other terrestrial mammals of comparable size. The respiratory quotient of six small-clawed otters with a mean body mass of 3.1 kg ± 0.4 kg at rest on land at 16 °C had a respiratory quotient of 0.77 and a resting metabolic rate of 5.0 ± 0.8 Wkg-1 (SD). This increased to 9.1 ± 0.8 Wkg-1 SD during rest in water and 11-15 °C, 17.6 ± 1.4 Wkg-1 during foraging in water at 12 °C (Borgwardt and Culik, 1999).
HABITATSIn most of their range the Asian small-clawed otters are sympatric with smooth-coated and Eurasian otters. In India all the three species occur in Southwest India, particularly in Western Ghats and possibly in the hills of Uttar Pradesh and Assam. In South India they are mostly found along hill streams. They were once common in the mangroves of east Calcutta and Sunderbans (Sanyal, 1988). In Malaysia and Indonesia including Java they occur in coastal wetlands, and along the banks of paddy fields. Comparable data from Java, Myanmar, and India revealed that the Asian small-clawed otters have a high climatic and trophic adaptability in South and Southeast Asian tropics, occurring from coastal wetlands up to mountain streams and in slow-flowing lowland streams to sub- montane streams dominated by rocks and boulders in forested areas. (Melisch et al., 1996). The typical natural habitats of small-clawed otter in west Java are wetland systems having pools and stagnant water, including shallow stretches with depth less than 1 m. These habitats are represented by freshwater swamps, meandering rivers, mangroves and tidal pools. Irrigated rice fields with many crab species (Brachyura sp.) are extensively used by small-clawed otters if proper shelter for them is available. These can act as suitable man made habitats (Melisch et al., 1996).
Habitat selection
Kruuk et al. (1994) found that although there were differences among Eurasian otter, smooth-coated otter and small-clawed otter in their macro-habitat, micro-habitat and food, there were overlaps between them. In Thailand, it occurs mostly in the middle sections and at the upper reaches of the rapid-flowing Huay Kha Khaeng River. When different otter species occurred in the same site there was evidence of a difference in use of the habitats. Signs of the small-clawed otter were found wandering further away from the river than the two other species, between patches of reeds and river debris where crabs were more likely to be found (Kruuk et al., 1994). In Eravikulum National Park its occupancy over smaller time scales indicated a strong time-dependant influence of altitude and habitat use intensity (Perinchery, 2008) and the intensity of its habitat use was influenced by altitude, followed by stream types, with pools preferred over cascades and riffles. Second and third order streams were used more intensively than first order streams (Perinchery et al., 2011).
In Annamalai hills the small-clawed otters occur in the Valparai plateau because the stream properties have not been fundamentally altered due to the presence of forest fragments, riparian vegetation. The human modified landscape is less used than the surrounding protected area because of human disturbance such as sand mining, fishing, and poaching (Prakash, 2010).
In west Java, the Asian small-clawed otters prefer pond areas and rice fields than the rivers, whereas they use mangroves and lakes in proportion to their availability. In riverine systems, they prefer moderate and low vegetation structure, though their presence was also observed from banks with poor vegetation cover (Melisch et al.,1996). Its presence is positively correlated with slow flowing and stagnant broad rivers and smaller streams, depicting a distinct preference decline from slow to deep-water bodies. On the other hand, they also use shallow fast-flowing mountain creeks narrower than 5 m, particularly when the course of the streams includes natural pools. In rice fields, they chose slow-flowing irrigation channels narrower than 2 m and with a varied, moderate or low vegetation structure. Like smooth-coated otter the Asian small-clawed otters dislike bare and open areas that do not offer any shelter (Melisch et al., 1996).
DIET/FEEDING
Otters have evolved two distinct foraging habits: piscivory and feeding on invertebrates. Piscivorous otters include Lutra species and the giant river otter Pteroneura brasiliensis. The invertebrate eaters include the clawless, small-clawed otters (Aonyx spp.) and the sea otter (Enhydra lutris) (Estes 1989). Like other Aonyx species such as Congo claw-less otter (A. congica) and Cape claw-less otter (A. capensis), the small-clawed otter is adapted to feed on invertebrates as evident from the last two upper teeth (pm4 and m3) which are larger in size for crushing the exoskeleton of crabs and other hard shelled prey. It feeds mainly on crabs, snails and other molluscs, insects and small fish such as gouramis and catfish (Pocock, 1941; Wayre, 1978). They supplement their diet with rodents, snakes and amphibians too. The quantitative information on the diet of small-clawed otter from different ecosystems is very scanty which has been reviewed in the subsequent sections.
Regional and seasonal variation in prey selection
During a study in Malaysia, Foster-Turley (1992) examined 328 spraints and found that around 80.8% of the diet of the small-clawed otter consisted of crabs, 77.8% fish, 12.5% insects and 4.0% snails. This is the first study in which quantitative information on the diet of wild Asian small-clawed otter was made. This study revealed that though it is adapted for an invertebrate diet it substantiates its diet with large quantities of fish. Apart from crabs, the major prey item for small-clawed otter was the mudskipper (Gobioidei sp.). The other important prey was Trichogaster sp. (20.7%), Anabantidae fish (27.4%), Anabis testudineus (5.2%), Clarius sp. (2.4%) and Channa striatus (1.5%). Apart from these they also consume snakes, frogs and insects in minor quantities.
In rice fields, the diet of the small-clawed otters was significantly different at different times of the year depending on water levels. Only the relatively rare dietary components of rodents, snails and snakehead fish (Clarius sp.) showed no significant difference among seasons. Crabs were always the most prevalent food items, but the frequency of occurrence in scats varied from 70.4% to 93.2%. Similarly, though the mudskippers were the second most important food items, they were consumed in significantly different amounts in different seasons from a low of 27.3% to 63.6%. The amount of Trichogaster sp., Anabas sp. and the entire family Anbantidae also varied considerably. This difference in the use of these prey items is most likely due to the difference in the life cycle and availability of these prey at different times of the year.
Preliminary analysis of the small-clawed otter spraints from west Java showed their preference for crabs in both natural and man-made habitats (Melisch et al., 1996). In 87% of all collected spraints, crabs formed the dominant prey. Remaining part of the spraints consisted of fish bones and scales, ribs and vertebrae, unidentified mammalian hair, shrimps, insects and snake scales.
In the Huay Kha Khaeng, Thailand almost 90% of the spraints of small-clawed otter contained remains of crabs Potamon smithianus, whereas 5% spraints contained each of Fish and Amphibians. Apart from this in few scats evidences of rodents and other arthropods were also found (Kruuk et al., 1994).
Size preference for major prey species
Kruuk et al. (1994) estimated the preference for various size classes of crabs eaten by small-clawed otter. Of the 92 spraints, 14 had crabs size 10-14 cm, 42 had 15-19 cm, 26 had 20-24 cm, 12 had 25-29 cm, four had 30-34 cm and one had 40-44 cm. The size distribution of crabs taken by small-clawed otter was similar to what was available, and there was not much evidence of selection for specific size. In west Java, a preliminary estimate of preferred size confirmed an average of 3-4 cm carapace width (Melisch et al., 1996).
REPRODUCTIONThe Asian small-clawed otter is amongst the least studied Asian otter species in wild. Information on its reproductive behaviour mostly comes from various zoos outside of its distribution range. Sexual behavior has been observed in pups as young as 6 months with breeding behavior having been noted in males and females as young as 18 months. Successful breeding has been reported for 2.1 year-old females and 2.8 year-old males. The youngest animal to reproduce was a 13 month-old female, captive born at Bronx Zoo, and the oldest was a male at the National Zoo, USA known to be more than 15 years old (Foster-Turley and Engfer, 1988).
Mating season
In the females oestrous cycle has duration of anywhere from 28 to 30 days, with breeding occurring the year round (Lancaster, 1975). Some facilities report this cycle extending to “every few months” with older animals. Oestrus lasts from one to thirteen days. Behavioral signs at the onset of oestrus may include increased rubbing and marking.
Mating behaviour
In captivity mating usually takes place in the water, but has also been observed on land on a few occasions. In most cases the exact gestation period could not be ascertained but it is believed to be around 60 days (Lancaster, 1975). Gestations of 62 to 86 days have been recorded in North American zoos. Sobel (1996) recorded a gestation length between 60 and 74 days from his study. Pregnancy is usually obvious by the increase in the females’ girth. The females collect grass and other materials such as hay or straw and carry it into the breeding chamber a few days before parturition. The males frequently help in the task of collecting bedding and carrying it into the maternity chamber (Lancaster, 1975).
Pregnancy
In Santa Barbara Zoo, urinary hormone levels in the females from six pairs of Asian small-clawed otters were compared with the observed reproductive behaviour during six oestrous periods. The study showed that regular peaks in progesterone corresponded with observed mating activities, both male and female engaged in nest building and sometimes females even showed signs of pregnancy; however no offspring were ever produced. Such results illustrate one of the most frustrating aspects of attempts to breed small-clawed otter (Foster-Turley and Engfer, 1988). Two litters can be produced in one calendar year (Foster-Turley and Engfer, 1988). During a study an inter-birth interval as short as eight months was observed (Sobel, 1996).
Litter size
There is no published information on the litter size in the wild. In captivity the litter size varies from 1-7 (n=16, mean=4.4) (Lancaster, 1975). In a recent compilation Lombardy and O'Connor (1998) concluded that the mean litter size was 3.5. (n=28). The observed pup sex ratio was not statistically different from an expected 1:1 ratio. Like all other otter species, the cubs are born with eyes closed that remain so until the fifth week. When they are about ten weeks old they make tentative exploratory excursions outside the breeding den. When about three months old they first enter and paddle in shallow water under the guidance and watchful eye of the mother. The cub survival is low in captivity. For instance only 54% of the 70 cubs born in Adelaide Zoo survived to independence (4-5 months) (Lancaster, 1975). At birth the weight of the pups ranges between 45.60-62.50 g to 410-988 g after 60 days (Maslanka and Crissey, 1998). Several studies in captivity clearly indicated that the Asian small-clawed otter lives in pair and both the parents take part in nest building and rearing of families (Leslie, 1970,1971; Timmins, 1971; Lancaster, 1975).
SOCIAL STRUCTURE
All that is known about the social behaviour of small-clawed otters is from studies in captivity. In captivity, they display a strong pair bond and both parents share the responsibilities of rearing the offspring. As many as seven cubs can be born in a litter. The inter-birth interval can be as short as ten months. Family groups can easily build up to 15 or more animals. In the wild groups up to 12 animals have been observed (Lekagul and McNeely 1977), which are mostly family groups.
COMMUNICATION
The small-clawed otter is capable of a wide range of sounds. Timmins (1971) recognized 12 or more distinct calls. They utter a variety of yelps and whimpers and when disturbed high-pitched ululating screams (Medway, 1969; Lekagul and McNeely, 1977). The distress call of small-clawed otter serves to rally the help of other otters (Sivasothi and Nor, 1994).
ACTIVITY PATTERN
The small-clawed otters are nocturnal and crepuscular particularly where they live near human habitation in Malaysia. At night their chirps can be heard in rice fields. They are occasionally seen in the early morning or around dusk (Foster-Turley 1992). In Malaysia, Foster-Turley (1992) observed that only small-clawed otter smeared their scat at the toilet sites. Scat smearing was found in 28 of the 63 positively identified small-clawed otter toilet sites. The difference in the scat smearing was not significantly different at various seasons. The presence of smeared scat was related to the size of the otter group. Among the larger groups smearing was more prominent. Groups of three or fewer animals rarely displayed smearing. The frequency of sites with smeared scats varied in different locations, indicating choice for certain sites. This scat smearing is most likely associated with scent-marking displays. The observation of increased scat smearing with larger groups may indicate the social facilitation of this behaviour (Foster-Turley 1992). Scat smearing in the shared area may be a scent-marking display with interspecific territorial implications (Foster-Turley 1992). In captivity, small-clawed otters are often observed in scat smearing displays. Typically, scat is smeared with the hind feet and the tail and all otter in the group participate in the activity.
RELATION WITH OTHER SPECIES
In most of their range the small-clawed otters occur sympatrically with crab-eating mongoose (Herpestes urva) and directly compete with them for food. The foraging behaviour and diet of crab-eating mongoose and small-clawed otter overlaps greatly. Apart from sharing their habitat with Eurasian and smooth-coated otters, the small-clawed otters also share their habitats with crab-eating mongoose. Other aquatic species such as Indian marsh crocodile or mugger(Crocodylus palustris), saltwater crocodile(Crocodylus porosus), Siamese crocodile(Crocodylus siamensis), and several species of turtles also occur sympatrically with them and compete in one way or other for the same resources.
THREATS
Throughout its range, the potential threat to the continued survival of the small-clawed otter is destruction of its habitats due to changing land use pattern in the form of developmental activities. In many parts of Asia, the habitats have been reduced due to reclamation of peat swamp forests and mangroves, aquaculture activities along the intertidal wetlands and loss of hill streams (Hussain et al., 2008). The primary threats to Asian small-clawed otter are loss of habitats due to tea and coffee plantations along the hills, siltation of smaller hill streams due to deforestation and in the coastal areas loss of mangroves due to aquaculture and increased human settlements (Sanyal, 1988) Increased influx of pesticides into the streams from the plantations reduces the quality of the habitats.
Other important threats to Asian small-clawed otter are reduction in prey biomass due to over-exploitation, which make its habitats unsustainable. Pollution is probably causing decline in the population of many fish species (Dehadrai and Ponniah, 1997). Reduction in prey biomass affects otter population, and organochloric and heavy metal contamination interferes with their normal physiology leading to a decline in population. The threat to small-clawed otter is prominent in its western range so much so that since last 60 years its range has shrunk considerably moving west to east from Himachal Pradesh to Assam. Once common in the mangroves of east Calcutta and Sunderbans (Sanyal, 1988), now it is believed to be locally extinct. It is likely that the present range boundary at the western limit is Assam and in the Western Ghats of South India. In many Southeast Asian countries it is regularly being killed, e.g rampant hunting and catching of these animals in Southern Palawan is persisting mainly because of curiosity and for food. Most of their habitats were already degraded with slash and burn farming that may cause the decline of their population. Because of their charismatic appeal they have a high public demand, leading to illegal trade in Palawan (Gonzalez, 2010).
RELATION WITH HUMANS
The Asian small-clawed otters are traditionally kept as pets not only in the Asian countries but also in many western countries. Besides being intricately woven with human culture the Asian small-clawed otters have significant economic value in terms of controlling crustacean populations in the rice fields which are otherwise destructive to rice culture in terms of destroying saplings and burrowing in the dykes leading to severe irrigation problems. At least eight species of crabs occur in rice fields of West Java. Some of these species also destroy mangrove saplings in the silviculture/plantation areas causing economic losses. Presence of small-clawed otters reduces such damages by controlling crustacean populations. On the other hand both the small-clawed and the smooth-coated otter damage paddy saplings because of their playful activities. They also prey upon rice field fish, which are otherwise an additional source of income for the farmers. They often raid fishponds and prawn farms causing large-scale damage, leading to human-animal conflicts.
CONSERVATION STATUS
It is considered “Vulnerable” under IUCN Red List due to an inferred future population decline because of habitat loss and exploitation (Hussain et al., 2008). In the last few decades the range of Asian small-clawed otter has shrunk particularly in its western portion (Hussain et al., 2008). The vulnerable status of the species based on past population decline rates under criterion A2acd. Given the extent of habitat loss that is occurring in South and Southeast Asia (Schipper et al., 2008; Hoffman et al., 2010) and the intensity of poaching, the reduction in population has been observed in many parts of its range (Hussain, 1993; Melisch et al., 1996; Hussain, 1999).
Conservation action
Since 1977, The Asian small clawed otter is listed on Appendix II of CITES which indicates that the species is not necessarily threatened with extinction, but the trade on its pelt must be controlled in order to avoid utilization incompatible with their survival. However, most range countries are not able to control the clandestine trade leading to extensive poaching. Nevertheless, it is a protected species in almost all the range countries which prohibits its killing. The Asian small-clawed otter once common in the streams and wetlands of South and Southeast Asia is now restricted to a few protected areas. Creation of networks of protected areas, identification of sites as wetland of national and International importance under Ramsar Convention has to some extent halted the degradation of its habitat.
Over the years the IUCN Otter Specialist Group has developed a cadre of biologist across Asia to conduct field surveys and has popularized otter conservation by promoting otter as ambassador of the wetlands. However, concerted effort to conserve this species is needed. For the long-term survival of the species, policy based action, research on factors affecting its survival, habitat based actions on creation and where required expansion of protected areas and communication and awareness building actions are needed. Basic studies on the habitat use, feeding ecology, interspecific interaction and resource partitioning with other aquatic species and its conservation genetics from different parts of its range covering different ecosystems will contribute to the conservation of this species.
Acknowledgements - This review is an updated version of a presentation given at the Xth International Otter Colloquium, October 10-17, 2007, Hwacheon, South Korea. The participation of corresponding author was supported by Dr. Sung-Yong Han, Organizing Secretary Xth IOC. We thank two anonymous reviewers for critically examining the manuscript and for suggesting improvement.
REFERENCES
Borgwardt, N., Culik, B.M. (1999). Asian small-clawed otters (Amblonyx cinerea): resting and swimming metabolic rates. Journal of Comparative Physiology B: Biochemical, Systematic and Environmental Physiology. 169: 100-106.
Corbet, G.B., Hill, J.E. (1992). The mammals of the Indomalayan region: a systematic review. Natural History Museum Publications, London. 488 pp.
Dehadrai, P.V., Ponniah, A.G. (1997). Conserving India’s fish Biodiversity. International Journal of Ecology and Environmental Sciences. 23: 315-326.
Duckworth, J.W. (1997). Small carnivores in Laos: A status review with notes on ecology, behaviour and conservation. Small Carnivore Conservation. 16: 1-21.
Estes, J.A. (1989). Adaptations for aquatic living by carnivores. In: Carnivore, behaviour, ecology and evolution. Ed. by John L. Gittleman. Dept. of Zoology and Ethology, University of Tennessee, Knoxville. London, Chapman and Hall. 620 pp.
Fish, F.E. (1994). Association of propulsive swimming mode with behaviour in the river otters (Lutra canadensis). J. Mammal. 75: 989-997.
Foster-Turley, P., Engfer, S. (1988). The species survival plan for the Asian Small-clawed Otter, Aonyx cinerea. Int. Zoo Yb., 27: 79-84.
Foster-Turley, P. (1992). Conservation ecology of sympatric Asian otters Aonyx cinerea and Lutra perspicillata. Ph.D. Dissertation, University of Florida. USA, 172 pp.
Foster-Turley, P., Santiapillai, C. (1990). Action plan for Asian otters, In Otters, an action plans for their conservation (Eds.) Pat Foster-Turley, Sheila Macdonald and Chris Mason. IUCN/SSC, Otter Specialist Group. IUCN, Gland. 126 pp.
Gonzalez, J.B. (2010). Distribution, exploitation and trade dynamics of Asian small-clawed otter Amblonyx cinereusIlliger 1815 in mainland Palawan, Philippines. A thesis submitted to the Western Philippines University Puerto Princesa Campus, Puerto Princesa City, Palawan. In partial fulfillment of the requirements for the degree of Bachelor of Science in Aquatic Biology. 58 pp.
Harris, C.J. (1968). Otters: A study of the Recent Lutrinae. London. Weidenffeld and Nicolson, United Kingdom. 307 pp.
Hoffmann, M., Hilton-Taylor, C., Angulo, et al. (2010). Theimpact of conservation on the status of the world's vertebrates. Science 330: 1503-1509.
Hussain, S.A. (1999). Species profile: Mustelids, Viverrids and Herpestids of India. ENVIS Bulletin on Wildlife & Protected Areas. Wildlife Institute of India. 2 (2): 1-38.
Hussain S.A. (1993). Aspects of the ecology of smooth-coated otter (Lutra perspicillata) in the National Chambal Sanctuary, India. Ph.D. Thesis, Centre for Wildlife and Ornithology, Aligarh Muslim University, Aligarh. 206 pp.
Hussain, S.A., de Silva, P.K. (2008). Aonyx cinerea. In 2008 IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/44166
Jefferies, D.J. (1989). The Asian short clawed otter Amblonyx cinerea (Illiger) living wild in Britain. Otters (Earsham), 2(3): 21-25.
Jefferies, D.J. (1991). Another record of an Asian short-clawed otter living free in Oxford with notes on its implications. J. Otter Trust, 2(5): 9-12.
Koepfli, K.P., Wayne, R.K. (1998). Phylogenetic relationships of otters (Carnivora: Mustelidae) based on mitochondrial cytochrome b sequences. J. Zool., London. 246: 401-416.
Koepfli, K-P., Kanchanasaka, B., Sasaki, H., Jacques, H., Louie, K. D. Y., Toanvong Hoai, Dang, N. X., Geffen, E., Gutleb, A., Han, S-Y., Heggberget, T. M., Lafontaine, L., Lee, H., Melisch, R., Ruiz-Olmo, J., Santos-Reis, M., Sidorovich, V. E., Stubbe, M., Wayne, R. K. (2008). Establishing the foundation for an applied molecular taxonomy of otters in Southeast Asia. Conservation Genetics 9: 1589-1604.
Kruuk, H., Kanchanasaka, B., O'Sullivan, S., Wonghogsa, S. (1994). Niche separation on the three sympatric otters (Lutra perspicillata, L. lutra, and Aonyx cinerea) in Huai Kha Khaeng, Thailand. Biological Conserv. 69: 115-120.
Lancaster, W.E. (1975). Exhibiting and breeding the Asian small-clawed otter at Adelaide Zoo. Int. Zoo Yearbook. 15: 63-65.
Lariviere, S. (2003). Mammal species. No. 72, American Society of Mammalogists, pp. 1-5.
Larson, S., Jameson, R., Etnier, M., Fleming, M., Bentzen, P. (2002). Loss of genetic diversity in sea otters (Enhydra lutris) associated with the fur trade of the 18th and 19th centuries. Molecular Ecology. 11: 1899-1903.
Lekagul, B., McNeely, J.A. (1977). Mammals of Thailand. Association for the Conservation of wildlife, Bangkok. 758 pp.
Leslie, G. (1971). Further observations on the oriental short-clawed otter Amblonyx cinerea at Aberdeen Zoo. International Zoo Yearbook. 11:112-13.
Leslie, G. (1970). Observations on the oriental short-clawed otter, Aonyx cinerea, at Aberdeen Zoo. International Zoo Yearbook. 10:79–81.
Lombardi, D. and O'Connor, J (1998). Asian small-clawed otter husbandry manual. 112 pp.
Maslanka, M.T., Crissey, S.D. (1998). Nutrition and diet. Pp. 1-18 in Lombardi, D., and J. O’Connor (eds.). Asian small-clawed otter (Aonyx cinerea) husbandary manual. Columbus Zoological Gardens and AZA Asian Small-Clawed Otter SSP. 22 pp.
Medway, L. (1969). The wild mammals of Malaya and offshore islands including Singapore. Oxford University Press, London, United Kingdom. 127 pp.
Melisch, R., Kusumawardhani, L., Asmoro, P.B., Lubis, I.R (1996). The otters of west Java-a survey of their distribution and habitat use and a strategy towards a species conservation programme. PHPA/Wetlands International - Indonesia Programme, Bogor.
Mucci, N., Arrendal, J., Ansorge, H., Bailey, M., Bodner, M., Delibes, Miguel., Ferrando, et al (2010). Genetic diversity and landscape genetic structure of otter (Lutra lutra) populations in Europe. Conservation Genetics 11: 583-599.
Perinchery, A. (2008). Conservation genetics of Indian otters and their habitat use in Eravikulam National Park. A Thesis Submitted to Manipal University In partial fulfillment for the degree of Master of Science in Wildlife Biology and Conservation. 61 pp.
Perinchery, A., Jathanna, D., Kumar, A.(2011). Factors determining occupancy and habitat use by Asian small-clawed otters in the Western Ghats, India. Journal of Mammalogy. 92 (4): 796-802.
Pertoldi, C., Hansen, M.M., Loeschcke, V., Madsen, A.B., Jacobsen, L., Baagoe, H. (2001). Genetic consequences of population decline in the European otter (Lutra lutra): an assessment of microsatellite DNA variation in Danish otters from 1883 to 1993. Proceeding of Royal Society London. 268:,1775-1781.
Pocock, R.I. (1941). The Fauna of British India including Ceylon and Burma. Vol. II. Taylor and Francis, London. 503 pp.
Pohl, H. (1920). Die Unterfamilie der Lutrinae (Eine systematisch-tiergeographische Studie an dem Material der Berliner Museen). Archiv für Naturgeschichte 85: 145-147.
Prakash, N. (2010). Factors influencing the occurrence and habitat use of small- clawed otters (Aonyx cinerea) in the human-modified landscape of Western Ghats, South India. A Thesis Submitted to theNational Centre for Biological Sciences, Tata Institute of Fundamental Research for the degree ofMaster of Science inWildlife Biology and Conservation. 40 pp.
Prater, S.H. (1948). The book of Indian animals. Second edition. Oxford University Press, Delhi. 324 pp.
Rafinesque, C.S. (1832). Description of a new otter Lutra concolor from Assam in Asia. Atlantic Journal. 1:62.
Roberton, S.I. (2007). The status and conservation of small carnivores in Vietnam. Ph.D. thesis. Centre for Ecology, Evolution & Conservation, School of Biological Sciences. University of East Anglia. U.K. 273 pp.
Sanyal, P. 1988. Status of otters in West Bengal, IUCN Asian Otter Spec. Group Newsletter, 1, 17.
Schipper, J., Chanson, J.S., Chiozza, F., Cox, N.A. et al. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science. 322: 225-230.
Sivasothi, N., (1996). The Small-clawed otter, Amblonyx cinereus Illiger, 1815 (Carnivora: Mustelidae: Lutrinae), the second resident wild carnivore in Singapore: Report of a family group on Pulau Tekong Besar and of the fate of wildlife there. The Pangolin, 7: 23-31.
Sivasothi, N., Nor, B.H.M. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia 285: 1-3.
Sobel, G. (1996). Development and validation of noninvasive, fecal steroid monitoring procedures for the Asian small-clawed otter, Aonyx cinerea. Master of Science Degree Thesis, University of Florida. 136 pp.
Sody, H.J.V. (1933). Ten new mammals from the Dutch East Indies. Annals Mag. Nat. Hist. 12: 430-442.
Timmins, W.H. (1971). Observations on breeding the oriental short clawed otter Amblonyx cinerea at Chester Zoo. International Zoo Yearbook. 11: 109-111.
van Zyll de Jong, C.G. (1987). A phylogenetic study of the Lutrinae (Carnivora: Mustelidae) using morphological data. Can. J. Zool. 65: 2536-2544.
van Zyll de Jong, C.G. (1991). A brief review of the systematics and a classification of the Lutrinae. In : Habitat. 6: 79-83.
Walker, E.P. (1975). Mammals of the World. Third edition. Baltimore, Johns Hopkins Press. 2015 pp.
Wayre, P. (1978) Status of otters in Malaysia, Sri Lanka and Italy. In Duplaix, N. (1978) Otter Trust Annual Report, 1974, pp. 152-155.
Willemsen, G.F. (1992). A revision of Pliocene and Quaternary Lutrinae from Europe. Scripta Geol., 101: 1-115.
Résumé : Etat des Lieux sur la Biololgie et L’Ecologie de la Loutre d’Asie Aonyx cinereus (Illiger, 1815)
La Loutre d'Asie est la plus petite des 13 espèces de loutres présentes dans le monde. Elle présente une très grande distribution, de l'Inde à l'Asie du sud-est jusque Taiwan et les Philippines à l'est puis la Chine méridionale au nord. Elle est classée comme espèce «vulnérable» en raison de la perte et de la dégradation des habitats, la raréfaction des proies et son exploitation. Son adaptation à la vie dans des ruisseaux peu profonds et les plans d'eau la rend plus vulnérable aux modifications de ses habitats par l’Homme ainsi qu’aux conséquences du changement climatique. Ce document résume l'état des connaissances sur la biologie et l'écologie de cette espèce peu connue. Au fil des ans, le Groupe de spécialistes de la Loutre de l'UICN a soutenu des biologistes à travers l'Asie afin de mener des enquêtes de terrain et de populariser la conservation de la Loutre en l’utilisant comme ambassadrice des zones humides. Toutefois, des efforts concertés sont nécessaires pour sauvegarder cette espèce. Pour sa survie à long terme, des actions politiques, des recherches sur les facteurs affectant sa survie, des actions de conservation des habitats, la création et/où l’extension d’aires protégées, la communication et des actions de sensibilisation sont nécessaires.
Revenez au dessus
Resumen: Revision De La Biologia Y Ecologia De La Nutria Inerme Asiática Aonyx cinereus (Illiger,1815)
La Nutria Inerme Asiática es la más pequeña de las trece especies de nutrias existentes. Su rango de distribución se extiende desde India en el sur de Asia; Taiwán y Filipinas en el este y el sur de China en el norte. Su estado de conservación se considera “Vulnerable” debido a la pérdida y degradación de su hábitat, disminución de sus especies presa y explotación. La especie está adaptada a vivir en esteros y cuerpos de agua de baja profundidad por lo que son vulnerables a la modificación de éstos, principalmente por impactos antropogénicos o como consecuencia del cambio climático. El presente artículo resume el estado de conocimiento acerca de la biología y ecología de esta especie. En los últimos años el Grupo de Especialistas en Nutrias de la UCIN SSC ha formado un conjunto de biólogos a lo largo de Asia los cuales han implementado censos y han popularizado la conservación de las nutrias promoviéndolas como “embajadoras de los humedales”. Sin embargo, mayores esfuerzos son necesarios para la sobrevivencia de la especie a largo plazo. Políticas de conservación y de investigación de las especies que se basen en estudios que identifiquen medidas para la sobrevivencia de esta nutria, protección del hábitat, expansión de áreas protegidas, así como la concientización de comunidades locales son necesarias
Vuelva a la tapa