IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 35 Issue 3 (October 2018)
Citation: Okamoto, Y., Jung, S-Y, Han, S-Y, Han, H-D, Shimizu, K, Kim, H-H, Eo, K-Y, Park, K-W, Oh, H-T, Park, K-W and Kimura, J (2018). Analysis of the Steroid Hormone Levels in the Eurasian Otter (Lutra lutra) by using Fecal Samples. IUCN Otter Spec. Group Bull. 35 (3): 159 - 170
Analysis of the Steroid Hormone Levels in the Eurasian Otter (Lutra lutra) by using Fecal Samples
Yumiko Okamoto1, So-Young Jung2, Sung-Yong Han3, Hyo-Dong Han2, Keiko Shimizu4, Hyeong-Hoo Kim3, Kyung-Yeon Eo2*, Keun-Woo Park2, Hyun-Taek Oh2, and Junpei Kimura5*
1 Hsinchu Zoo: 279, Kung-Yung Rd, Hsinchu City, Taiwan 300. y81kgd@yahoo.co.jp
2 Seoul Grand Park: Daeongwongwangjang-ro 102, Daegongwongwangjang-ro, Gwacheon-shi, Gyeonggi-do, 427-704, Korea. vetinseoul@seoul.go.kr
3 Korean Otter Research Center: Hwacheon 209-802, Korea. hsy5034@hanmail.net
4Okayama University of Science: 1-1, Ridai-cho, Kita-ku, Okayama city, 700-0005, Okayama, Japan. shimizu@zool.ous.ac.jp
5Seoul National University: 599 Gwanangno, Gwanak-gu, Seoul, 151-742, Korea. jay.kimura@mac.com
Received 30th November 2017, accepted 26th February 2018
Abstract: The population of the Eurasian otter has declined due to habitat destruction, pollution and urbanization. Studying reproductive physiology is one of the most essential things for conservation. However, there is no report which aims to understand basic reproductive physiology of this species in Korea. Hence, fecal samples of 6 Eurasian otters kept in Korea were collected for 7-12 months to analyze steroid hormone levels to understand their basic reproductive physiology. It was succeeded to identify individual feces by fecal markers for the first time in this species in Korea. A young female otter showed clear increases in fecal estradiol and progesterone levels and both of hormone levels were much higher than that of a subadult and old female otters. The average of interval period of each peak in progesterone of a young female was 43.5 days. Pseudopregnancy was not observed. Fecal testosterone level of an old male otter was as high as that of a young male otter. However, fecal testosterone level of a subadult male otter was very low. Steroid hormone levels of otters rose between January and November in this study, which mostly coincides with a study that reports the reproductive season of Eurasian otters in Korea is from January to September. Attempt was successful in monitoring endocrine traits by using fecal samples and breeding season of Eurasian otters in Korea. This study represents the first comprehensive examination of endocrine traits of Eurasian otters in Korea, and these findings may contribute to the conservation and management of this species.
Keywords: breeding season, Eurasian otter, fecal steroids, noninvasive monitoring
INTRODUCTION
The Eurasian otter (Lutra lutra) is distributed in Eurasia and Northern parts of Africa (Sjoasen, 1995, Lee, 1996, Ruiz-Olmo et al., 2001, Kruuk, 2006, IUCN, 2014). The population of the Eurasian otter, however, has been decreasing rapidly in recent decades due to habitat destruction, pollution or urbanization (Sjoasen, 1995, Lee, 1996, Koelewijn, 2010). The Eurasian otter is currently designated as a Near Threatened species on the IUCN Red List and listing on Appendix Ⅰ of the Convention on international trade in endangered species (CITES) (IUCN, 2014, CITES, 2014). Among East Asian countries, this situation is much more serious. In Japan, it was declared in August 2012 that the Japanese otter (Lutra lutra nippon) has already become extinct (Kruuk, 2006, Ando, 2008, IUCN, 2014, Kushimoto, 2014), and the number of Eurasian otters in China and Taiwan is declining (Lee, 1996, Li, 2005, Piao et al., 2011). The population of the Eurasian otter in Korea has also declined rapidly during the last several decades even though it had once been abundant throughout the country (Ando, 2008, Kim et al., 2011, Seo et al., 2014). Therefore, Eurasian otters in Korea were designated as Natural monument No.330 in 1982 and Korean endangered species I in 2004 in order to conserve them, and several studies about the population of Eurasian otters in Korea have been carried out (Ando, 2008, Kim et al., 2011, Han and Yoon, 2012).
With wild otter populations in crisis, captive otters have become more important as a research resource to better understand basic otter biology and as a potential genetic reserve for otter conservation (Bateman et al., 2009). In particular, a comparative data set of basic reproductive information also would benefit development of reproductive technologies, such as artificial insemination and genome resource banking, as population management tools (Bateman et al., 2009). Through prior studies, several basic reproductive characteristics of the Eurasian otter were revealed. The age of sexual maturity of this species seems to be quite variable (Melissen, 2000), however, it was assumed that most of Eurasian otters produce their first litter by the age of 2 years (Kruuk et al., 1991). Furthermore, there is no delayed implantation in the Eurasian otter (Sandell, 1990). After a gestation period of about 63 days, on average 2-3 cubs are born (Kruuk, 2006). Eurasian otter cubs may be born at any time of the year (Heggberget and Christensen, 1994, Melissen, 2000), however, in the wild, seasonal birth peaks of the Eurasian otter may vary among different parts of habitat range, probably related to the seasonality of food resources (Kruuk, 1991, Heggberget and Christensen, 1994, Kruuk, 2006).In Korea, it was reported that the reproductive season of wild Eurasian otters as starting from the end of January and ending at the end of September (Han, 1997).
In many nondomestic species, reproductive endocrine traits may be characterized, without requiring frequent capture and anesthesia, by using noninvasive monitoring of urine or fecal metabolites (Schwarzenberger, 2007, Bateman et al., 2009, Kummrow et al., 2010). For example, reproductive characteristics of the Small-clawed otter (Aonyx cinereus), the North-American river otter (Lontra canadensis) and the Sea otter (Enhydra lutris) were studied by using their fecal samples (Larson et al., 2003, Bateman et al., 2009). In the Eurasian otter, steroid hormone levels of fecal samples collected in the wild were analyzed to know the population structure in the specific area (Kalz et al., 2006) and the effect of the environmental hormone to reproduction (Jansman et al., 2001). Also, there is a paper which aims to understand basic reproductive physiology of the Eurasian otter in captivity (Tschirch et al., 1996). Therefore, steroid hormone levels of Eurasian otters in Korea were analyzed for the first time by using fecal samples to understand reproductive characteristics in this study.
Although to get individual feces without contamination is very important for the fecal hormone analysis, zoo exhibits are not generally designed with ease of sample collection (Fuller et al., 2011). A common approach to this problem is to feed animals fecal markers that subsequently appears in feces (Fuller et al., 2011). It was succeeded to get individual feces by using this method in the Small-clawed otter (Aonyx cinereus) and the North American River Otter (Lontra canadensis) (Fuller et al., 2011) and in the Eurasian otter (Grohmann and Klenke, 1996, Kalz et al., 2006). Hence, fecal marker was used for the hormone analysis in this study as other reports.
MATERIALS AND METHODS
For fecal hormone analysis, a total of 4 adult and 2 subadult Eurasian otters housed at Seoul grand park (Seoul Zoo) and Korean otter research center (KORC) were assessed in this study. All otters were born in the wild and rescued within 2 months after they were born, therefore, only rescued date or estimated birth date are known (Table 1). Of these 6 Eurasian otters, 1 male and 2 females were paired for breeding, whereas the remaining 2 males and 1 female were housed alone. Although husbandry programs differed between two institutions, the feeding of Eurasian otters were generally comprised of Channel catfish (Ictalurus punctatus), Weather loach (Misgurnus anguillicaudatus), Horse mackerel (Trachurus japonicus) or Landlocked salmon (Oncorhynchus masou masou). Feeding was offered from one to three times per day.
| Table 1: The individual characteristics of captive Eurasian otters used in this study. | |||||
| Number | Name | Sex | Facility | Birth/Rescued Date |
Reproductive History |
| 1 | Hyo-joo | F | Korean Otter Reserach Centre | 2011.06.11 (Rescued) | None |
| 2 | Bok soon-i | F | Korean Otter Reserach Centre | 2013.09.(Estimated birth) | None |
| 3 | Busan | F | Seoul Zoo | 2004.12 (Rescued) | None |
| 4 | Jindal | M | Korean Otter Reserach Centre | 2010.06 (Rescued) | Once* |
| 5 | Joon-ki | M | Korean Otter Reserach Centre | 2013.06 (Rescued) | None |
| 6 | Samcheok | N | Seoul Zoo | 2003.09.01 (Rescued) | None |
| *A female otter kept with Jindal (No.4) became pregnant in August 2013 | |||||
Fecal samples were collected noninvasively after natural voiding from once to fifth per week for time spans ranging from 7 months to 1 year. For animals housed in pairs, fecal samples were identified by feeding one or more individuals nontoxic glitter powders (Glitter powder-Metal Color, Igonji, Seoul, Korea) mixed in the diet and collecting only samples containing that specific marker. Fecal samples were transferred into a labeled plastic bag and immediately stored frozen at -20 °C.
Fecal Marker
To get the individual feces of No.3, nontoxic colored glitter powder was put into the Horse mackerel and it was given to No.3 consecutively with regular feedings. The glitter particles were less than 1mm in diameter and did not affect food intake or digestion of the Eurasian otter.
Fecal sample extraction
First, samples were dried at -85 °C for 24-48 hours in the freeze drier (FDU-2100, EYELA, Tokyo, Japan). After drying, 0.2g of fecal powder in a 17×120mm tube containing 2ml of phosphate buffered saline and 10ml of ether were vortexed for 1min. Then samples were shaken slowly on the dancer machine (Stovall Belly Dancer Shaker, Cole-Parmer, Illinois, USA) for 10min and centrifuged at 3×100rpm, 4 °C for 10min by a centrifugal separator (TOMY MX-301, Tomy Seiko, Tokyo, Japan). For extracting hormone metabolites, samples were stored frozen at -75 °C for 24hr. Next day, ether liquid was evaporated completely by using nitrogen gas. Therefore, 1ml Tris-HCl was added into the each tube with hormone metabolite, and all these tubes were vortexed for 1 min and stored in the refrigerator at -30°C until the analysis.
Fecal Hormone Analysis
Time-resolved Fluoroimmunoassays (FIA) Progesterone
Fluoroimmunoassay Progesterone analyzing kit (DELFIA Progesterone kit, PerkinElmer, Massachusetts, USA) was used to analyze Progesterone levels of Eurasian otters in this study. First, 25 μl standards and samples were put into the each well, and 100 μl tracer dilution and antiserum dilution were added. Then, the plate was incubated on the slow shaking plate shaker for two hours. The plate was washed off by the plate washer (1296-026 DELFIA Platewasher, PerkinElmer, Massachusetts, USA) and 200 μl enhance liquid was put into the plate. The plate was shaked slowly on the plate shaker for five minutes. At last, the concentration of Progesterone was calculated by the plate reader (Victor 2D, Perkin Elmer, Massachusetts, USA). According to the manufacture instructions, the sensitivity of the DELFIA Progesterone assay was better than 0.00025ng/ml and the cross-reactivities tested by Progesterone FIA were as follows: Progesterone 100%, 5β-Dihydroprogesterone 90.0%, 5α-Dihydroprogesterone 13.0%, Corticosterone 2.4%, 17α-Hydroxyprogesterone 1.26%, Pregnanolone 0.80%, 11-Deoxycortisol 0.12%, 11-Deoxycorticosterone and 20α-Dihydroprogesteron <0.1%.
Time-resolved Fluoroimmunoassays (FIA) Estradiol
Fluoroimmunoassay Estradiol analyzing kit (DELFIA Estradiol kit, PerkinElmer, Massachusetts, USA) was used to analyze Estradiol levels of Eurasian otters in this study. First, the plate was washed off by a plate washer. After washing, 25 μl standards and samples were put into the each well and 100 μl antiserum dilution was added. Then, the plate had been incubated on the slow shaking plate shaker for thirty minutes and 100 μl trace dilution was put into the plate. The plate was shaked and incubated on the plate shaker for two hours. After incubation, the plate was washed off by the plate washer and 200 μl enhance was put into the plate. Then, the plate was shaked slowly on the plate shaker for five minutes. At last, the concentration of Estradiol was calculated by the plate reader. According to the manufacture instructions, the sensitivity of the DELFIA Estradiol assay was better than 0.0136ng/ml and the-cross reactivities were as follows: Estradiol-17β 100%, 16-Oxoestradiol 1.8%, Estrone 1.5%, Estradiol-3-glucuronide 1.1%, Estriol 0.8%, Estradiol-3-sulphate 0.28%, 16-Hydroxyestradiol 0.08%, Estrone-3-sulphate 0.04%, 2-Hydroxyestradiol 0.04%.
Enzyme Immunoassay (EIA) Testosterone
First, 50µl of Testosterone anti-body was put into the each well and the assay plate was incubated overnight at room temperature. After incubation, the assay plate was washed 3 times by the plate washer (MR-96CR, BioTek, Tokyo, Japan). 50µl of assay buffer was put into all wells, and the assay plate was shaken slowly for 30min on the plate shaker (NR-10, TAITEC, Koshigaya, Japan). 50µl standards, controls and samples were added to each well. Then, 50µl Testosterone-HRP were added to each well and the assay plate was incubated overnight at room temperature. The assay plate was washed 3 times by the plate washer after incubation and 100µl of OPD substrate were added to each well. Then, the assay plate was shaken fast for 30min on the plate shaker. At last, 50µl of stop solution was added into each well and the concentration of Testosterone was calculated by reading absorbance at the plate reader (Sunrise rainbow, TECAN, Mannedorf, Switherland). The sensitivity of this assay was better than 0.015ng/ml and the cross-activities were as follows: Testosterone 100%, 5-Dihydrotestosterone 7.0%, 4-Androstenedione 2.0%, Androsterone 0.2%, 5-Androstene-3B, 17B-diol 0.15%, 5a-Androstane-3a, 17B-diol 0.10%, 5B-Androstane-3a, 17B-diol, Cortisol 0.02%, Corticosterone, Pregnenolone, Progesterone, 17a-Hydroxypregenolone, Aldosterone, Dehydroepiandrosterone and Estradiol <0.01%.
RESULTS
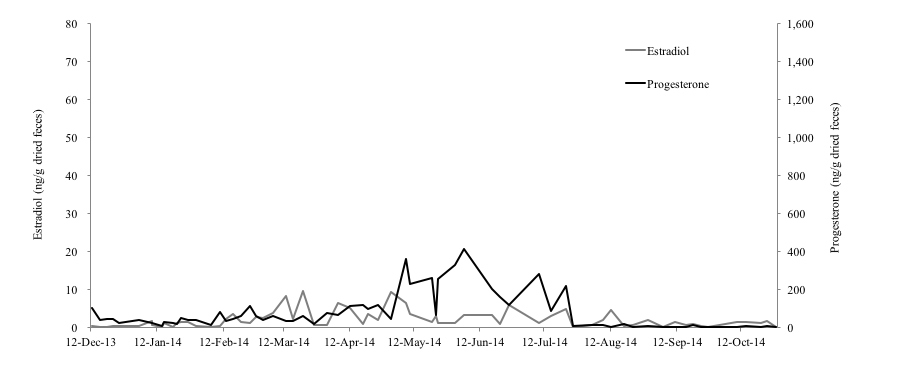
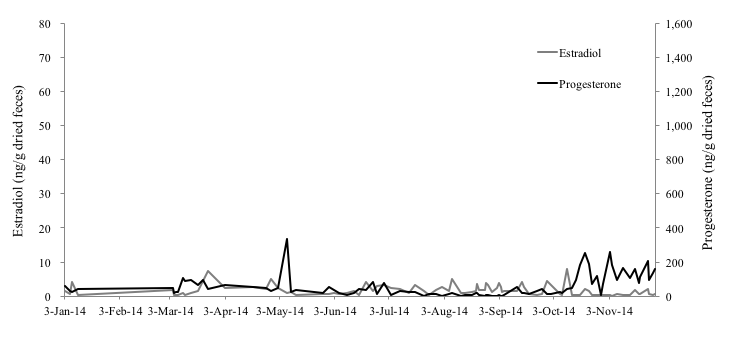
Of the three female Eurasian otters monitored for this study, the 4-year-old female otter (No. 1) showed clear increases on February 24th, April 4th and May 4th in fecal estradiol levels and on February 27th, April 4th and May 24th in fecal progesterone levels (Figure 1). Both of hormone levels of this individual were 0.17-67.28 ng/g and 6.15-1441.26 ng/g, respectively. The average of interval period of each peak in progesterone of No.1 was 43.5 days. Compared to the results of No.1, both of the hormone levels of the 1-year-old (No. 2) and 10-year-old female (No. 3) otters were very low and no clear increase was observed (Fig. 2, 3). The fecal estradiol and progesterone levels were 0.18-9.74 ng/g and 2.65-411.34 ng/g for No.2, and 0.08-7.89 ng/g and 1.98-337.55 ng/g for No.3, respectively. None of the Eurasian otters became pregnant. Although the fecal estradiol and progesterone levels of No.1 and No.2 became higher from January-February to August-October, the progesterone level of No. 3 rose slightly only from the beginning of October to the end of November.
Horse mackerels with glitter powder were eaten by No. 3 without hesitation, allowing for the collection of uncontaminated fecal samples. Furthermore, No. 3 did not experience any health problems during this study.
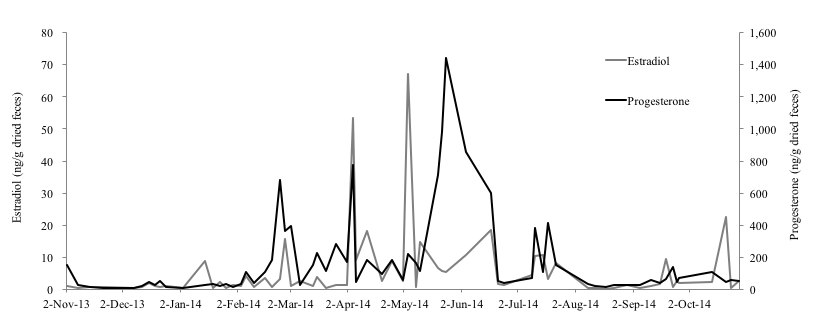
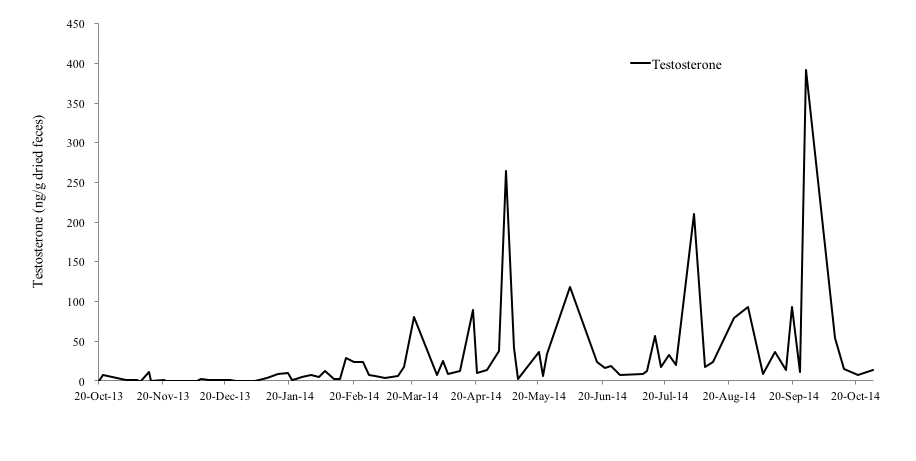
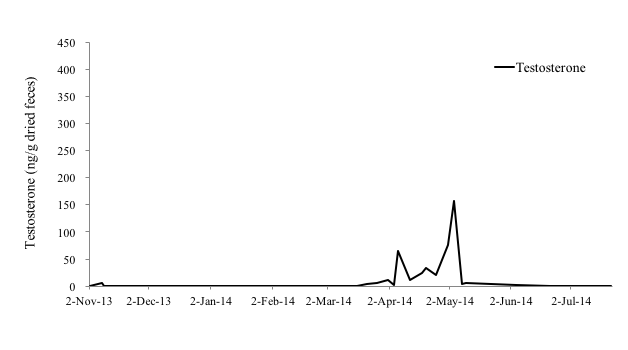
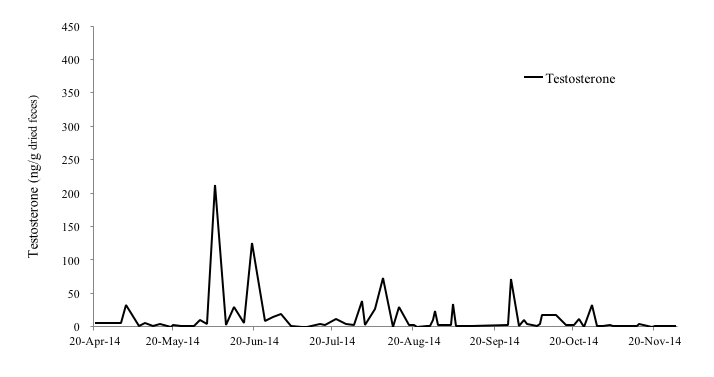
Among the male otters, the fecal testosterone levels of the 4-year-old male (No. 4), 1-year-old male (No. 5) and 11-year-old male otter (No. 6) were 0.25-391.64 ng/g, 0-157.45 ng/g, and 0.00-211.67 ng/g, respectively (Figure 4-6). In particular, No. 4 and No. 6 showed clear increases in fecal testosterone levels from March-May to September-October. However, the fecal testosterone level of No. 5 rose slightly only from April to May, and its overall level was very low compared to No. 4 and No. 6.
DISCUSSION
In mammalian female animals, ovarian activities (follicular growth-ovulation-formation of corpus luteum) are repeated after reaching sexual maturity. The blood concentration of estradiol increases during the follicular growth period, because the dominant follicle produces great amount of estradiol. Then, corpus luteum produces substantial progesterone (Schillo, 2009). The estradiol level of No. 1 rose significantly in the beginning of April and May, and its progesterone level rose significantly three times: at the end of February, the beginning of April, and the end of May. Therefore, it was thought that follicles grew and ovulation occurred at least two times during this study. Although no ovulation occurred in No. 2, the growth of follicles seems to have been occurred from February to August and this ovarian activity indicated that this subadult female otter was reaching sexual maturity. It was shown that fecal estradiol and progesterone levels of No. 1 were much higher than No. 2 and No. 3. Otters produce their first litter by the age of 2 years, as they become sexually mature during their second year (Kruuk et al., 1991). Therefore, it was thought that the low hormone level of No.2 could be explained by the fact that the age of this individual was under the breeding age. There is a report that the average life span of otters in the wild is 4 to 5 years (Melissen, 2000). However, another hormonal study on two 17-year-old female Eurasian otters found that, while the fecal estradiol level of one was very low, that of the other 17-year-old female was as high as younger otters (Nishimori, 2014), which results show that the ovary of the latter was still working actively (Nishimori, 2014). In addition to that, it was reported that the average life span of captive Eurasian otters is 12 to 14 years and the longevity record in captivity belongs to a female individual that was 18.2 years of age when it died (Weigl, 2005). Hence, it can be suggested that female Eurasian otters in captivity can join reproduction much longer than wild otters. The result of low hormone level of No.3 can be related to the old age, however, the reproductive status of a female Eurasian otter differs depending on its individual conditions, more researches are necessary to reveal it.
In the case of pregnancy, progesterone is produced by the corpus luteum or placenta to maintain the pregnancy (Schillo, 2009). In addition to that, some mammalian species show high progesterone concentrations after copulation without pregnancy, which is called pseudopregnancy (Schillo, 2009). It is said that, while pseudopregnancy occur in the North American river otter and the Small-clawed otter (Bateman et al., 2009), it does not seem to occur in the Eurasian otter (Nishimori, 2014). Any female Eurasian otter did not show high concentration of progesterone for long period, therefore, pseudopregnancy was also not observed in this study.
In this study, the fecal estradiol and progesterone levels of No. 1 and No. 2 rose from January-February to August-October, which mostly coincides with the results of the study that report that the reproductive season of wild Eurasian otters in Korea starts from the beginning of January and ends at the end of September (Han, 1997).
It was confirmed that glitter powder has no effect on the health of the Eurasian otter, and this fecal marker was effective in collecting individual feces from otters in captivity without contamination. Although the uses of fecal markers for hormone analysis on the Eurasian otter were already reported (Tschirch et al., 1996, Kalz et al., 2006), it was also successful to identify individual feces in this study.
It was revealed that the elevation of fecal estradiol levels coincided with copulation behavior in the Sea otter (Larson et al., 2003). However, the elevation of fecal estradiol and androstenedione levels had no relevance to reproductive behavior in the Eurasian otter (Nishimori, 2014). Reproductive behavior such as copulation, vocalization or chasing could not be observed in this study because the installation of surveillance cameras in the otter enclosures for the sole purpose of this study was not allowed.
It was reported that the fecal testosterone levels of the small-clawed otter were correlated with seasonal changes in serum testosterone concentrations and testicular volume (Bateman et al., 2009). Hence, it was thought that the fecal testosterone levels of the Eurasian otter also indicate serum testosterone concentrations and testicular activity (Nishimori, 2014). In the Black-footed Ferret (Mustela nigripes), testes and seminal quality are indistinguishable among males from 1 to 5 years old ages, with progressive reproductive aging occurring thereafter (Wolf et al., 2000), however, age influence to the production of testosterone in the Eurasian otter is unclear. A study reported that the fecal testosterone levels of a 26-year-old male Eurasian otter was higher than that of a 7-year-old male otter (Nishimori, 2014). In this study, the concentrations of fecal testosterone of a 11-year old male otter (No. 6) was similar with that of a 4-year old male otter (No.4). Therefore, it was assumed that the influence of aging on fecal testosterone levels is negligible in the Eurasian otter.
As mentioned before, otters produce their first litter by the age of 2 years as they become sexually mature during their second year (Kruuk et al., 1991). Hence, it was believed that the fecal testosterone level of the 1-year old male otter (No. 5) was very low because this otter had not yet reached sexual maturity. Sperm is produced in male Small-clawed otters when their testosterone level is elevated (Bateman et al., 2009). However, it was reported in male cynomolgus monkeys (Macaca fascicularis) that fecal testosterone levels can be affected by other reasons like social rank stress (Czoty et al., 2009). In this study, all male individuals are not kept with any other male otters, it seems that stress effect is not so big. Therefore, it can be suggested that the elevation of fecal testosterone levels of male Eurasian otters also indicates the testes are active. In this study, the concentration of fecal testosterone of No. 4 and No. 6 rose from March-May to September-October and that of No. 5 rose from April to May, which also supports the claim that the reproductive season of wild Eurasian otters in Korea spans the beginning of January to the end of September (Han, 1997). Hence, it was thought that male Eurasian otters in Korea are also seasonal breeders, and their breeding season coincides with female otters.
A longitudinal assessment of fecal progesterone, estradiol, and testosterone has proven useful for understanding the reproductive physiology of the Eurasian otter. The reproductive status of otters such as the presence of ovulation and pseudopregnancy, sexual maturity, and breeding season could be monitored. However, pregnancy and estrus behavior could not be observed in this study, and the sample size was also relatively small compared to other hormone analyzing studies using wild animals in captivity (Larson et al., 2003, Bateman et al., 2009). Difficulties in securing a large sample set stemmed from physical and logistic complications such as the size of the enclosures, the clinical death of an otter, and the transfer of otters to other institutions.
This study represents the first comprehensive examination of endocrine traits of Eurasian otters in Korea, and these findings may contribute to the conservation and management of this species. However, further studies are necessary to improve endocrine monitoring of steroid hormones to further explore pregnancy, age of sexual maturity, ovulation mechanism and, the relationship between steroid hormones and estrus behavior in the Eurasian otter.
Acknowledgements: We would like to thank all those who assisted to conduct this study. First, in Korea, special thanks go to Jun-Woo Choi and Jin-Cheon Kim in KORC. In Japan, Keiko Mouri and Misato Hirai in Okayama university of science.
REFERENCES
Ando, M. (2008). The Japanese otter: lessons from its extinction. University of Tokyo Press, Tokyo. [In Japanese].
Bateman, H.L., Bond, J.B., Campbell, M., Barrie, M., Riggs, G., Snyder, B., Swanson, W.F. (2009). Characterization of basal seminal traits and reproductive endocrine profiles in North American River Otters and Asian Small-Clawed Otters. Zoo. Biol. 28: 107-126.
CITES Appendix, (2014). Appendices I, II and III.
Czoty, P., Gould, R., Nader, M. (2009). Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J. Neuroendocrinol. 21(1): 68-76.
Fuller, G., Margulis, S.W., Santymire, R. (2011). The effectiveness of indigestible markers for identifying individual animal feces and their prevelence of use in North American Zoos. Zoo Biol. 30: 379-398.
[Note: the North American River Otter is identified erroneously as Lontra felina]
Grohmann, O., Klenke, R. (1996). Farbmarkierte Nahrung. Methodische Aspekte und praktische Beispiele zur Raumnutzung. Freistaat Sachsen, Dresden.
Han, C W., Yoon, M.H. (2012). Construction Works at the Busan New Port on the Activity of Otters. Korean J Ecol Environ. 26: 654-667. [In Korean]
Han, S. Y. (1997). The Ecological Studies of Eurasian otter (Lutra lutra) in South Korea. Kyungnam University. Ph. D Thesis. [In Korean]
Heggberget, T. M., Christensen, H. (1994). Reproductive timing in Eurasian otters on the coast of Norway. Ecography 17: 339-348.
IUCN Red List of Threatened Species, (2014).
Available via http://www.iucnredlist.org/. Cited on February 2014.
Jansman, H. A. H., Bosveld, A. T. C, Van Dan, B. C., Niewold, F. J. J. (2001). Genetical and hormonal monitoring of otters Lutra lutra by analysis from spraints. Wiss. Mitt. Niederosterr. Landesmuseum, 14: 129-130.
Kalz, B., Jewgenow, K., Fickel, J. (2006). Structure of an otter (Lutra lutra) in Germany – results of DNA and hormone analyses from faecal samples. Mamm. Biol. 71: 321-335.
Kim, H., Ando, M., Han, S., Sasaki, H., Ogawa, H. (2011). Recovery of the Eurasian otter Lutra lutra in Korea and the change in public attitude. IUCN Otter Spec. Group Bull. 28B: 85-90.
Koelewijn, H.P., Perez-Haro, M., Jansman, H.A.H., Boerwinkel, M.C., Bovenschen, J., Lammertsma, D.R., Niewold, F.J.J., Kuiters, A.T. (2010). The reintroduction of the Eurasian otter (Lutra lutra) into the Netherlands: hidden life revealed by noninvasive genetic monitoring. Conserv. Genet., 11: 601-614.
Kummrow, M.S., Gilman, C., Mackie, P., Smith, D.A., Mastromonaco, G.F. (2011). Noninvasive analysis of fecal reproductive hormone metabolites in female veiled chameleons (Chamaeleo calyptratus) by enzyme immunassay. Zoo. Biol., 30: 95-115.
Kushimoto, T. (2014). The Skull of the Eurasian otter found in the coast of Hutaoi island, Shimonoseki. Bull. Firefly Museum of Toyota Town. 6: 19-22. [In Japanese]
Kruuk, H. (2006). Otters ecology, behavior and conservation. Oxford University Press, NY.
Kruuk, H., Conroy, J.W.H., Moorhouse, A. (1991). Recruitment to a population of otters (Lutra lutra) in Shetland, in relation to fish abundance. J. Appl. Ecol. 28: 95-101.
Larson S., Casson, C.J., Wasser, S. (2003). Noninvasive reproductive steroid hormone estimates from fecal samples of captive female sea otters (Enhydra lutris). Gen. Comp. Endocrinol., 134: 18-25.
Lee, L.L. (1996). Status and distribution of river otters in Kinmen, Taiwan. Oryx 30: 202-206.
Li, F., Chan, P.L. (2017). Past and present: the status and distribution of otters (Carnivora: Lutrinae) in China. Oryx 1-8.
Li, Y. (2005). Conservational study on two species of otter in Hainan Island, China. The research financially report supported by Pro Natura Fund, 157-162. [In Japanese]
Melissen, A. (2000). Husbandry guidelines EEP/Studbook for Lutra lutra. Otter Park Aqualutra, Netherlands.
Nishimori, S. (2014). The research of reproductive physiology by using steroid hormone metabolites of fecal samples in the Eurasian otter (Lutra lutra). Gifu University. Bachelor’s degree thesis. [In Japanese]
Piao, Z., Sui, Y., Wang, Q., Li, Z., Niu, Li. (2011). Population fluctuation and Resource Protection of Otter (Lutra lutra) in Changbai Mountain Nature Reserve. J. Hydrol., 32: 115-120.
Ruiz-Olmo, J., Olmo-Vidal, J.M., Manas, S., Batat, A. (2001). The influence of fish abundance on the otter (Lutra lutra) populations in Iberian Mediterranean habitats. J Zool. 254: 325-336.
Sandell, M. (1990). The Evolution of Seasonal Delayed Implantation. QRB, 65: 23-42.
Schillo, K.K. (2009). The Reproductive Physiology of Mammals. Delmar Cengage Learning, NY.
Seo, H., Shin, Y., Lee, K., Kim, Y., Jeon, M., Nam, S., Han, S. (2014). Vegetation Structure in Otter (Lutra lutra) Home Range of Hwacheon-gun, Gangwon-do. KJEE, 47: 66-73. [In Korean]
Schwarzenberger, F. (2007). The many uses of non-invasive feacal steroid monitoring in zoo and wildlife species. Int. Zoo Yearb., 41: 52-74.
Sjoasen, T. (1995). Survivorship of captive-bred and wild-caught reintroduced European otters (Lutra lutra) in Sweden. Biol. Conserv., 76: 161-165.
Tschirch, W., Hempel, G., Rothmann, H., Schipke, R., Klenke, R. (1996). Artenschutzprogramm Fischotter in Sachsen. Methodische Aspekte und praktische Beispiele zur Raumnutzung. Freistaat Sachsen, Dresden.
Weigl, R. (2005). Longevity of mammals in captivity; from the living collections of the world. Kleine Senckenberg-Reihe 48, Stuttgart.
Wolf, K.N., Wildt, D.E., Vargas, A., Marinari, P.E., Kreeger, J.S., Ottinger, M.A., Howard, J.G. (2000). Age dependent changes in sperm production, semen quality, and testicular volume in the black-footed ferret (Mustela nigripes). Biol. Reprod., 63: 179-187.
Analyse des Concentrations en Stéroïdes chez la Loutre Eurasienne (Lutra Lutra) à l’Aide d’Échantillons Fécaux
La population de loutre eurasienne a diminué à cause de la destruction de son habitat, de la pollution et de l’urbanisation. L’étude de la physiologie de la reproduction est une des priorités essentielles pour la conservation. Cependant, il n’existe aucun rapport qui a pour objectif de comprendre la physiologie reproductive de base de cette espèce en Corée. A cette fin, des échantillons fécaux de 6 loutres ont été prélevés durant 7 à 12 mois afin d’analyser les concentrations en hormones stéroïdiennes et comprendre la physiologie reproductive de base. Nous avons réussi pour la première fois à identifier des épreintes individuelles à l’aide de marqueurs fécaux sur cette espèce en Corée. Une jeune femelle a montré une nette augmentation de la concentration en estradiol fécal et en progestérone et ces deux concentrations en hormone étaient bien plus élevées que celles d’un subadulte et de femelles âgées. La moyenne de la période d’intervalle entre chaque pic de progestérone chez une jeune femelle était de 43,5 jours. Une pseudo gestation n’a pas été observée. La concentration en testostérone d’un vieux mâle était plus élevée que celle d’un jeune mâle. Cependant, la concentration en testostérone fécale d’un mâle subadulte était très basse. Les concentrations en hormones stéroïdiennes des loutres ont augmenté entre janvier et novembre dans cette étude, ce qui coïncide, la plupart du temps, avec une étude qui rapporte que la saison de reproduction de la loutre eurasienne se situe entre janvier et septembre. L’essai d’un suivi des caractéristiques endocriniennes a été couronné de succès grâce à l’utilisation d’échantillons fécaux durant la saison de reproduction de la loutre eurasienne en Corée. Cette étude représente la première analyse complète des caractéristiques endocriniennes de la loutre eurasienne en Corée et ces résultats pourront contribuer à la conservation et à la gestion de cette espèce.
Revenez au dessus
Resumen: Análisis de los Niveles de Hormona Esteroidea en la Nutria Euroasiática (Lutra Lutra) utilizando Muestras Fecales
La población de nutria euroasiática ha declinado debido a destrucción de hábitat, contaminación y urbanización. El estudio de la fisiología reproductiva es una de las cosas más esenciales para la conservación. Sin embargo, no existen estudios dirigidos a entender la fisiología reproductiva de esta especie en Korea. Por ende, colectamos muestras fecales de 6 nutrias eurosiáticas en cautiverio en Korea, durante 7-12 meses, para analizar los niveles de hormona esteroidea, para entender su fisiología reproductiva básica. Tuvimos éxito identificando fecas individuales mediante marcadores fecales por primera vez para esta especie en Korea. Una hembra joven mostró claros aumentos en los niveles de estradiol y progesterona fecal, y ambos niveles hormonales fueron mucho más altos que el de un subadulto y una hembra vieja. El promedio del período de intervalo entre picos de progesteerona en una hembra joven fue de 43.5 días. No observamos seudo-preñez. En un macho viejo encontramos un nivel de testosterona fecal tan alto como el de un macho joven. Sin embargo, el nivel de testosterona fecal de un macho subadulto era muy bajo. En este estudio, los niveles de hormona esteroidea de las nutrias subieron entre Enero y Noviembre, lo que mayormente coincide con un estudio que informa que la estación reproductiva de la nutria euroasiática en Korea es de Enero a Septiembre. Nuestro intento de monitorear los rasgos endocrinos y la estación reproductiva en Korea utilizando muestras fecales, fue exitoso. Este estudio representa el primer examen abarcativo de los rasgos endocrinos de las nutrias en Korea, y estos hallazgos pueden cntribuir a la conservación y manejo de esta especie
Vuelva a la tapa









