IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 37 Issue 4 (December 2020)
Citation: Palei, N.C., Rath, B.P., Palei, H.S. and Acharya, B.P. (2020). Population Status and Activity Pattern of Smooth-Coated Otter (Lutrogale perspicillata) in Bhitarkanika National Park, Odisha, Eastern India IUCN Otter Spec. Group Bull. 37 (4): 205 - 211
Population Status and Activity Pattern of Smooth-Coated Otter (Lutrogale perspicillata) in Bhitarkanika National Park, Odisha, Eastern India
Nimain Charan Palei1, Bhakta Padarbinda Rath1, Himanshu Shekhar Palei2, and Bimal Prasanna Acharya3
1Office of the Principal Chief Conservator of Forests (Wildlife) and Chief Wildlife Warden, Odisha, India
1 Department of Zoology, North Orissa University, Baripada, Takatpur, Odisha, India
3 Divisional Forest Officer, Mangrove Forest Division (WL), Rajnagar, Odisha, India
Corresponding Author: Email: wildpalei@gmail.com
(Received 22nd February 2020, accepted 23rd May 2020)
Abstract: The smooth-coated otter Lutrogale perspicillata is an IUCN-Vulnerable species as a result of habitat loss and poaching. The objective of this study was to estimate the population status and activity pattern of smooth-coated otter in Bhitarkanika National Park. To achieve this, 15 camera trap stations were established in the study area between 16th June and 5th August 2019.We recorded notionally 30 independent capture events of smooth-coated otter, over a total of 725 camera trap days. The encounter rate of smooth-coated otter was 2.06/photo-captures/100 trap days, with a diurnal activity pattern. Further research and monitoring, and awareness campaigns for local stakeholders is required in order to design effective conservation strategies of the species.
Keywords: Lutrogale perspicillata, Camera trapping, Kernel density, mangrove Forest, coastal area, Bhitarkanika National Park
INTRODUCTION
The smooth-coated otter Lutrogale perspicillata (Geoffroy), a medium sized otter weighing between 7 and 11 kg (Prater, 2005) is distributed throughout southern Asia from Indonesia, through Southeast Asia, and westwards through southern China, Pakistan and India, with an isolated population in Iraq (Pocock, 1941; Hussain, 1993; de Silva et al., 2015). In India, it is widely distributed from the foothills of Himalayas southward to southern India occurring in major rivers and coastal areas (Prater, 2005; Hussain, 1993). Smooth-coated otter is semi-aquatic social carnivore depending upon wetland habitats, which are currently among the most threatened and vanishing ecosystems worldwide (Davidson, 2014). Poaching for pelt and retaliatory killing as a result of otter-human conflicts are detrimental to the survival of this species across its distributional range (de Silva et al., 2015). Therefore, it has been categorized as ‘Vulnerable’ in the IUCN Red List of Threatened Species (de Silva et al., 2015) and legally protected under Schedule II Part II of the Indian Wildlife (Protection) Act, 1972.
In Odisha, three species of otters have been reported earlier viz; the smooth-coated otter, Eurasian otter Lutra lutra and the Asian small-clawed otter Aonyx cinereus (Illiger) (Acharjyo, 1999; Mohapatra et al., 2014; Adhya and Dey, 2020). Although, smooth-coated otter’s distribution covers most parts of the state of Odisha, ecological data of the species in the state are not available. This lack of ecological data may be due to the often elusive behaviour or typically low density of the species (Kruuk, 2006), or probably due to the lack of targeted surveys which is evident recent faunal inventories (Debata et al., 2015; Palei et al., 2018a; Debata et al., 2018; Kar et al., 2018). In recent years, camera trapping is increasingly used for species inventories and population status estimation, especially of cryptic species (Karanth and Nichols, 1998; Datta et al., 2008; Rovero and Marshall, 2008) and has been widely used in the state of Odisha as well (Palei et al., 2015; Debata and Swain, 2018; Palei et al., 2018b; Palei et al., 2019a,b).
In order to improve existing knowledge about smooth-coated otter across the range, the objective of this study was to determine the population status and activity pattern of the species in Bhitarkanika National Park, Odisha, eastern India. To achieve this goal, we conducted a camera trap survey in Bhitarkanika National Park.
STUDY AREA
We conducted the study in the Bhitarkanika National Park (BhitarkanikaNP; 20° 30' – 20° 48' N; 86° 45' – 87° 03' E), a mangrove forest area of 145 km2 in the state of Odisha, Eastern India (Fig. 1). It is characterized by rich alluvial deposits of the Brahmani, Baitarani, and Dhamra rivers. The major habitat type of the study area includes mangrove vegetation, creeks, estuaries, mudflats and water bodies. The area receives an average annual rainfall of 1680 mm, with minimum and maximum monthly temperature variations between 15 ºC and 40 ºC. The climate is humid tropical coastal with pronounced dry(hot) and wet seasons. The land elevation ranges from3.66 m to 8.23 m. Bhitrakanika NP has a wide network of rivers and creeks, which are mainly fed by tidal water. The major vegetation associations along the creeks consists of mangrove species, such as Heritiera fomes Buch.-Ham., Sonneratia apetala Buch.-Ham, Avicennia officinalis Linnaeus and Excoecaria agallocha Linnaeus.
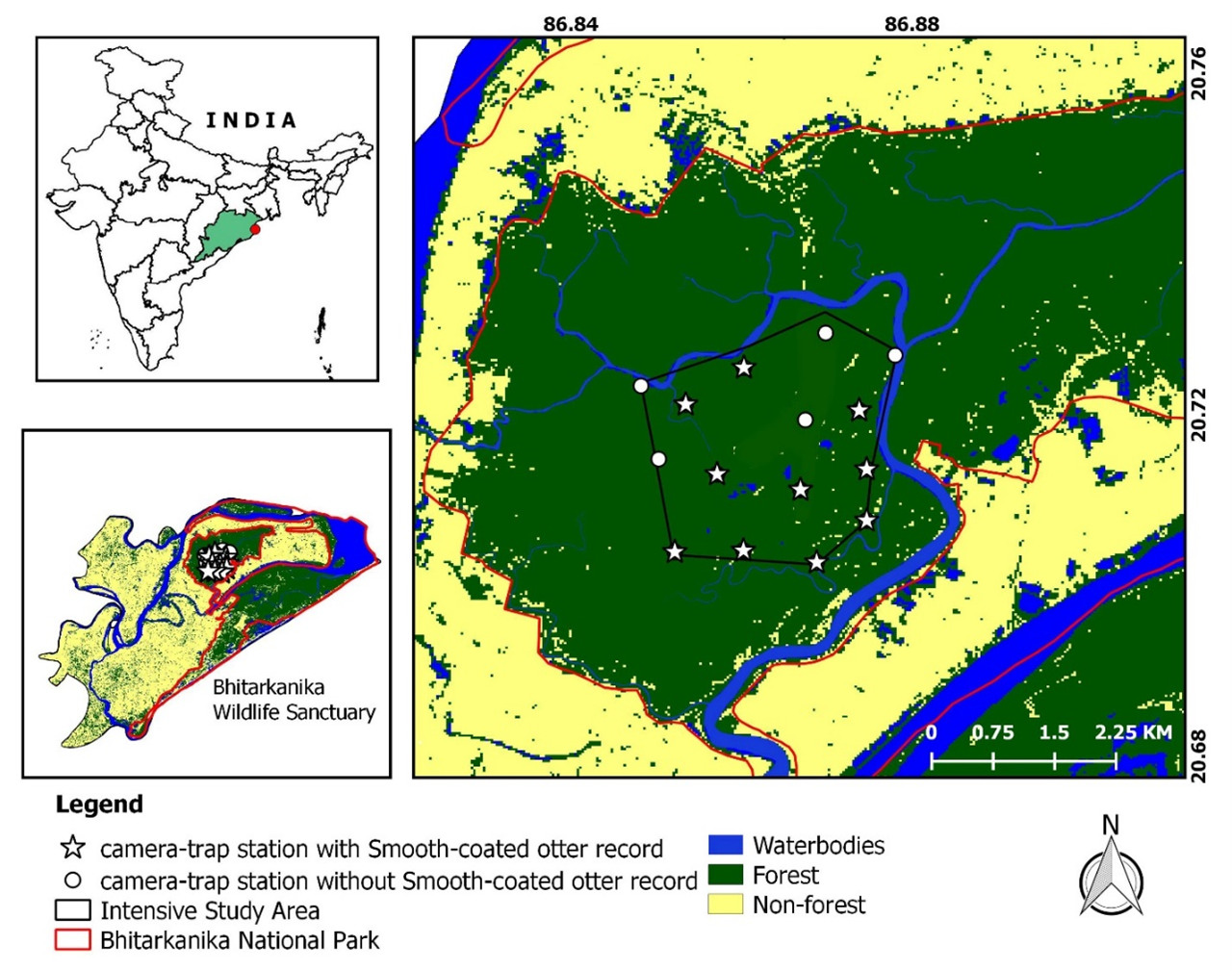
The area is a home to more than 24 species of mammals (Venkatraman et al., 2016), 264 species of birds (Gopi and Pandav, 2007) and 51 species of herpetofauna comprising 37 species of reptiles and 14 species of anurans (Jena et al., 2013;Venkatraman et al., 2016).
METHODOLOGY
Camera trap survey was carried out from 16th June to 5th August 2019 as a part of a broader study of mammalian diversity. The survey was conducted within the “intensive study area” (ISA) of 10 km2, representing all major habitat found in the Bhitarkanika NP (Fig. 1). We divided the ISA into 1 km2 grids and systematically choose grids for camera locations based on preliminary sign surveys. We deployed 30 camera traps in 15 locations with the nearest-neighbour distance between them at least 1 km in the ISA to ascertain the status of the mammal. We selected most suitable camera trap locations (otter trails, near waterbodies and along the water’s edge of creeks) which are likely to allow the camera-trapping of animals based on preliminary sign surveys. Camera traps were strapped to trees or stakes approximately 40 cm above ground. In this survey, all cameras were operational 24 hours per day for 50 days. No baits or lures were used in any camera trap station during the survey. Cameras were checked every week to replace the batteries and memory cards and to ensure their proper functioning. Total sampling effort was calculated as the sum of the effective days across all stations that each camera was functioning (Boitani and Powell, 2012). We considered photos separated by at least 30 minutes as notionally independent events (Ohashi et al., 2013; Guo et al. 2017). Photos more than one individual of smooth-coated otter in the frame were counted as one detection for the species.
To determine the local status of smooth-coated otter, the encounter rate is expressed as the number of notionally independent picture events/total sampling effort x 100. Kernel-density estimation was used to describe temporal activity of smooth-coated otter. This method considers each photographic record as a random sample of an underlying continuous distribution, instead of grouping photographic records in blocks of predefined discrete time categories (Ridout and Linkie, 2009). The analysis was performed using package “overlap” implemented in R version 3.5.1. (Meredith and Ridout, 2017; R Development Core Team 2019).
RESULTS
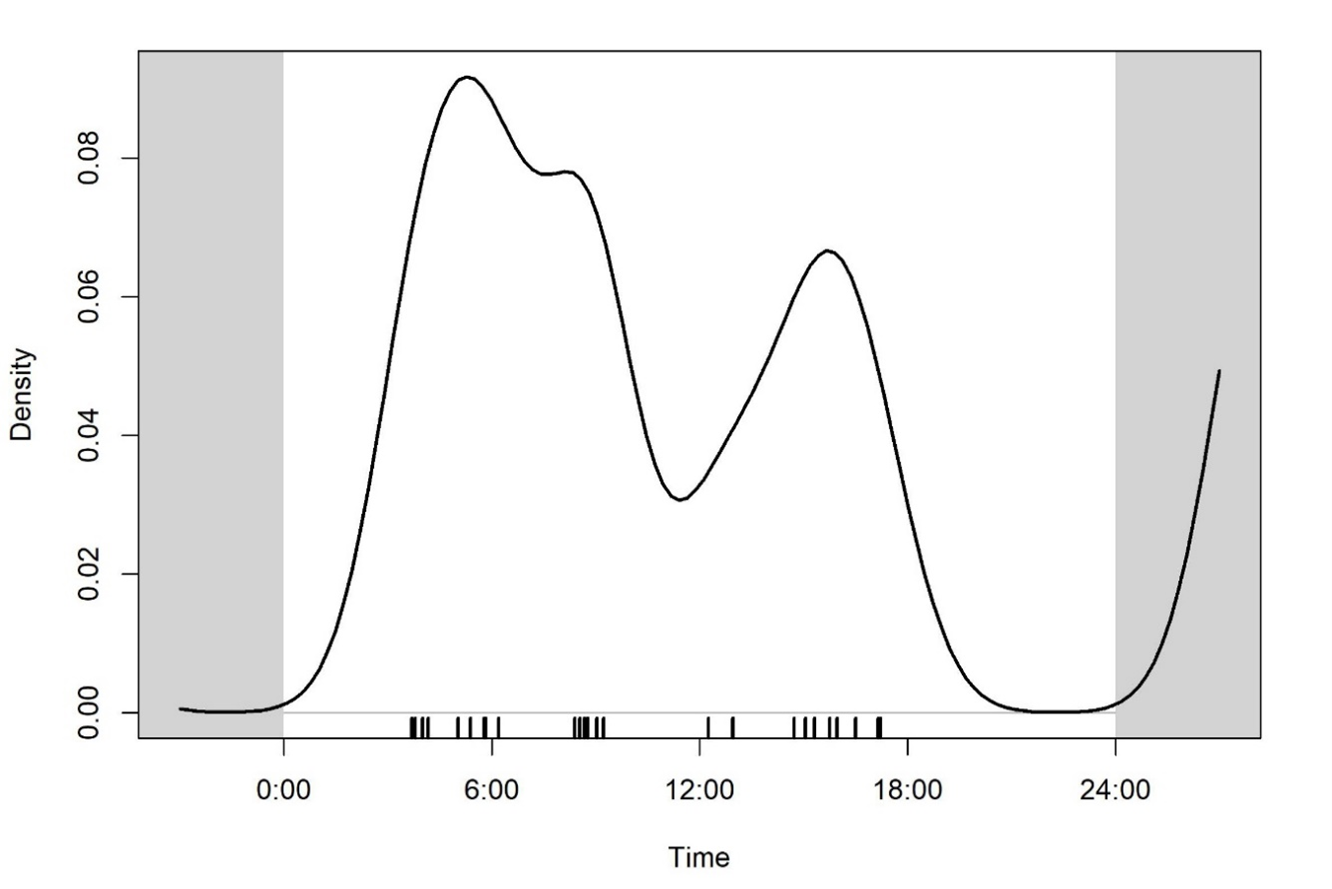
The sampling effort totaled 725 camera trap days. Smooth-coated otter was detected at 10 of the 15 sites (67%) at least once during the sampling period in total of 30 notionally independent events with at least one individual (Fig. 2). The encounter rate of smooth-coated otter was equal to 2.06 events/100 camera trap days. Smooth-coated otter was mostly diurnal, with more actively between 06:00 and 18:00 (67% of the notionally independent movement) with a peak around morning (Fig. 3). Smooth-coated otter showed bimodal peaks in its activity; the first peak was observed from early morning to mid-day and the second smaller was in the late afternoon. Though smooth-coated otters were active throughout the day they exhibited reduced activity during the hottest hours of the day (Fig. 3).

DISCUSSION
To our knowledge, this is the first camera trap based data on encounter rate and activity patterns of smooth-coated otter in its range. Therefore, it is difficult to compare this encounter rate with those from any other studies. Smooth-coated otters exhibited a diurnal and crepuscular activity pattern that peaked around early morning in our study area. Similar observation was reported for river otter Lontra canadensis (Schreber, 1777) in Canada, where otters were active throughout the day, but with bimodal peaks during the early morning and late evening hours (Martin et al., 2010). However, our results differ from those from National Chambal Sanctuary, India where the annual activity pattern of smooth-coated otters were substantially nocturnal (Hussain, 2013). Several factors may affect the abundance and activity pattern in otters, such as predators, prey availability, anthropogenic disturbance, or quality and type of habitat (Hussain, 2013). The absence of large predators like tiger Panthera tigris (Linnaeus) and leopard P. pardus (Linnaeus) and very low anthropogenic disturbance in Bhitarkanika NP may explain the high level of day-time smooth-coated otter activity there.
Our data should be used as baseline information for making future management and conservation strategies of the species in Bhitarkanika NP. The rapidly changing, human-dominated landscape poses the major threat to smooth-coated otter survival through range reduction and fragmentation (de Silva et al., 2015). Therefore, further detailed research should focused on smooth-coated otter beyond protected areas in order to have better understanding and management of the species in human dominated landscape.
Acknowledgements: We are thankful to the Principal Chief Conservator of Forest (Wildlife) and Chief Wildlife Wardens, Odisha for support in carrying out the study. We are also thankful to Divisional Forest Officer, Mangrove Forest (Wildlife) Division, Rajnagar and Range Officer, Kanika Wildlife Range and his staff for their unfailing extended help during the fieldwork. We thank the editor and anonymous reviewers for valuable discussions and comments that significantly improved the quality of manuscript.
REFERENCES
Acharjyo, L.N. (1999). Status of Mustelids, Viverrids and Herpestidsof Orissa. In: Envis Bulletin on Wildlife and Protected Areas. Hussain, S.A. (Eds) WII, Dehradun, 2: 62–64.
Adhya, T., Dey, P. (2020). First record of Eurasian otter (Lutra lutra) from chilika lagoon: a Ramsar site situated on the east coast of India. OTTER, Journal of the International Otter Survival Fund, 6: 49–55.
Boitani, L., Powell, R.A. (2012). Carnivore ecology and conservation: a handbook of techniques. Oxford University Press.
Datta, A., Anand, M.O., Naniwadekar, R. (2008). Empty forests: Large carnivore and prey abundance in Namdapha NationalPark, north-east India. Biological Conservation,141: 1429–1435.
Davidson, N.C. (2014). How Much Wetlands has the World Lost? Long-term and Recent Trends in Global Wetland Area. Marine and Freshwater Research, 65(10): 934–941.
de Silva, P., Khan, W.A., Kanchanasaka, B., Reza Lubis, I., Feeroz, M.M., Al-Sheikhly, O.F. (2015). Lutrogale perspicillata. The IUCN Red List of Threatened Species 2015: e.T12427A21934884. Downloaded on 19 February 2020.
Debata S., Palei H.S., Mohapatra P.P., Palita S.K. (2015). Additional Records of Cantor’s Leaf-Nosed Bat HipposiderosgaleritusCantor, 1846 (Mammalia: Chiroptera: Hipposideridae) in Eastern India: Odisha. Journal of Threatened Taxa,7(8): 7477–7479.
Debata S., Swain K. K. (2018). Estimating mammalian diversity and relative abundance using camera traps in a tropical deciduous forest of Kuldiha Wildlife Sanctuary, eastern India. Mammal Study, 43: 45–53.
Debata, S., Kar, T., Swain, K.K., Palei, H.S. (2018). The vulnerable Indian Skimmer Rynchops albicollis Swainson, 1838 (Aves: Charadriiformes: Laridae) breeding in Odisha, eastern India. Journal of Threatened Taxa, 9: 10961–10963.
Gopi, G.V., Pandav, B. (2007). Avifauna of Bhitarkanika Mangroves, India. Zoos’ Print Journal, 22(10): 2839–2847.
Guo, W., Cao, G., Quan, R.-C. (2017).Population dynamics and space use of wild boar in a tropical forest, Southwest China. Global Ecology & Conservation, 11: 115–124.
Hussain, S.A. (1993). Aspects of the ecology of smooth-coated otters Lutra perspicillata in National Chambal Sanctuary. Unpublished Ph.D Thesis. Centre for Wildlife and Ornithology. Aligarh Muslim University. Aligarh, India.
Hussain, S.A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth-coated otter (Lutrogale perspicillata) in National Chambal Sanctuary, India. IUCN Otter Specialist Group Bulletin, 30(1): 5–17.
Jena, S.C., Palita, S.K., Mahapatra, M.K.(2013). Anurans of Bhitarkanika mangroves, Odisha, east coast of India. Check List, 9(2): 400–404.
Kar, T., Palei, H.S., Debata, S. (2018). Breeding reports and conservation implications of the Endangered Black-bellied Tern Sterna acuticauda J.E. Gray, 1831 (Aves: Charadriiformes: Laridae) in Odisha, eastern India. Journal of Threatened Taxa, 10(13): 12840–12843.
Karanth, K.U., Nichols, J.D.(1998). Estimation of tiger densities inIndia using photographic captures and recaptures. Ecology,79: 2852–2862.
Kruuk, H. (2006). Otters: ecology, behaviour, and conservation. Oxford University Press Inc., New York.
Martin, D.J., Mcmillan, B.R., Erb, J.D., Gorman, T.A., Walsh, D.P. (2010). Diel activity patterns of river otters (Lontra canadensis) in south-eastern Minnesota. Journal of Mammalogy, 91(5):1213-1224.
Meredith, M., Ridout, M. (2017). Overview of the Overlap Package. R project.
Mohapatra, P. P., Palei, H.S., Hussain, S.A. (2014). Occurrence of Asian Small-Clawed Otter Aonyx cinereus (Illiger, 1815) in Eastern India. Current Science, 107(3): 367–370.
O’Brien, T.G., Kinnaird, M.F., Wibisono, H.T. (2003). Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation, 6: 131–139.
Ohashi, H., Saito, M., Horie, R., Tsunoda, H., Noba, H., Ishii, H., Kuwabara, T., Hiroshige, Y., Koike, S., Hoshino, Y., Toda, H., Koichi, K. (2013). Differences in the activity pattern of the wild boar Sus scrofa related to human disturbance. European Journal of Wildlife Research, 59: 167–177.
Palei, H.S., Das, U.P., Debata, S. (2018a). The vulnerable fishing cat Prionailurus viverrinus in Odisha, eastern India: status and conservation implications. Zoology and Ecology, 28(2): 69–74.
Palei, H.S., Palei, N.C., Rath, B.P., Mishra, A.K. (2019a). Records of the globally threatened rusty-spotted cat in Odisha, India. Nature Conservation Research, 4(3): 112–116.
Palei, H.S., Pradhan, T., Sahu, H.K. and Nayak, A.K. (2015). Estimatingmammalian abundance using camera traps in the tropical forest of Similipal Tiger Reserve, Odisha, India. Proceedings of the Zoological Society, 69: 181–188.
Palei, N.C., Rath B.P., Palei H.S., Mishra A.K. (2018b). Occurrence of melanistic leopard in Odisha, eastern India. Cat News, 68: 7–8.
Palei, N.C., Rath, B.P., Palei, H.S., Mishra, A.K. (2019b). Photographic evidences of Indian grey wolf (Canis lupus pallipes) in Sundargarh forest division, Odisha, India. e-planet, 17(2): 152-156.
Pocock, R.I. (1941). The fauna of British India, including Ceylon and Burma. Volume 2, Mammals, pp. 265–317. Taylor and Francis, London.
Prater, H.S. (2005). The Book of Indian Animals. New Delhi: Oxford University Press.
R Development Core Team. (2019). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. www.R-project.org.
Ridout, M.S., Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural Biological and Environmental Statistics, 14: 322–337.
Rovero, F., Marshall, A.R. (2008). Camera trapping photographicrate as an index of density in forest ungulates. Journal of Applied Ecology,46: 1011–1017.
Venkatraman, C., Padmanaban, P., Shrinivaasu, S., Sivaleela, G. (2016). Faunal diversity of Bhitarkanika mangroves, Odisha. Records of the Zoological Survey of India, 116: 407–430.
Statut de la Population et Profil d’Actvite de la Loutre à Pelage Lisse (Lutrogale perspicillata) dans le Parc National de Bhitarkanika, à Odisha, dans l’Est de l’Inde
La loutre à pelage lisse Lutrogale perspicillata est classée comme espèce vulnérable par l’UICN en raison de la perte d’habitat et du braconnage. L’objectif de cette étude était d’estimer l’état de la population et le schéma d'activité de la loutre à pelage lisse dans le parc national de Bhitarkanika. Pour ce faire, 15 pièges photographiques ont été installés dans la zone d’étude entre le 16 juin et le 5 août 2019. Nous avons enregistré théoriquement 30 événements indépendants de loutres à pelage lisse, sur un total de 725 jours de piégeages photographiques. Le taux de rencontre de la loutre à pelage lisse était de 2,06 / captures-photo / 100 jours de piégeages, avec un modèle d’activité diurne. Des recherches et un suivi supplémentaires, ainsi que des campagnes de sensibilisation des décideurs locaux sont nécessaires afin de concevoir des stratégies de conservation efficaces pour l’espèce.
Revenez au dessus
Resumen: Status Poblacional y Patrones de Actividad de la Nutria Lisa (Lutrogale perspicillata) en el Parque Nacional Bhitarkanika, Odisha, India Oriental
La nutria lisa Lutrogale perspicillata es una especie catalogada como Vulnerable por la UICN como resultado de pérdida de hábitat y caza furtiva. El objetivo de este etudio fue estimar el status poblacional y los patrones de actividad de la nutria lisa en el Parque Nacional Bhitarkanika. Para lograrlo, fueron establecidas 15 estaciones de cámaras-trampa en el área de estudio, entre el 16 de Junio y el 5 de Agosto de 2019. Registramos 30 eventos de captura fotográfica de nutria lisa -conceptuados como independientes-, sobre un total de 725 días-cámara-trampa. La tasa de encuentro con nutrias lisas fue de 2.06 foto-capturas/100 días-trampa, con un patrón de actividad diurno. Se requiere más investigación y monitoreo, y campañas de sensibilización de los actores locales, para diseñar estrategias de conservación efectivas para esta especie.
Vuelva a la tapa



