IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 2 (March 2021)
Citation: Andeska, F., Novarino, W., Nurdin, J. and Aadrean (2021). Relationship between Temporal Environment Factors and Diet Composition oOf Small-Clawed Otter (Aonyx cinereus) in Heterogeneous Paddy Fields Landscape in Sumatra, Indonesia. IUCN Otter Spec. Group Bull. 38 (2): 106 - 116
Relationship between Temporal Environment Factors and Diet Composition of Small-Clawed Otter (Aonyx cinereus) in Heterogeneous Paddy Fields Landscape in Sumatra, Indonesia
Ferdi Andeska, Wilson Novarino, Jabang Nurdin, and Aadrean*
Biology Department of Andalas University, West Sumatra, Indonesia.
* Corresponding Author: e-mail aadrean@sci.unand.ac.id
Received 24th August 2020, accepted 28th October 2020
Abstract: The small-clawed otter (Aonyx cinereus) is one of four otter species in Indonesia. This species consumes various types of animals. The availability of prey animal depends on its environmental conditions. Therefore, this study aimed to examine relationship between temporal environmental factors and diet composition of the small-clawed otter in paddy field landscape. We examined 415 spraints collected from a heterogeneous paddy field landscape in West Sumatra, Indonesia. We found that small-clawed otter consumed fishes, snails, crabs, reptiles, frogs, birds, and mammals. Unlike in the natural habitat, fish became the dominant diet rather than crabs. Furthermore, we performed generalized linear model (GLM) analysis to explain the temporal environmental factors that affect composition of small-clawed otter’s diet. GLM analyses revealed that temperature, rainfall, water level and cultivation stage were not significantly related to fish composition in small-clawed otter diet. Water level showed positive relationship to snails in the diet composition. Temperature showed positive relationship to both insect and frogs in the diet composition.
Keywords: Generalized Linear Model, Paddy field cultivation stage, Score-Bulk estimate, Opportunist predator
INTRODUCTION
The small-clawed otter (Aonyx cinereus) is one of four otter species in Indonesia. The small-clawed otter is adapted to the tropical climates of South and Southeast Asia (Melisch et al., 1994). This species can be found in freshwater and brackish water, including swamp forests, rice fields, lakes, streams, reservoirs, waterways, mangroves, and along the coast (Sivasothi and Nor, 1994). The small-clawed otter is listed in The International Union for Conversation of Nature’s Red List as a Vulnerable animal. (Wright et al., 2015). Since November 2019, the status of the small clawed otter in CITES is upgraded from Appendix II to Appendix I (Okamoto et al., 2020).
Small-clawed otters consume various types of animals: fish, crabs, molluscs, amphibians, insects, reptiles, birds, and mammals (Foster-Turley, 1992; Kruuk et al., 1994; Anoop and Hussain, 2005; Hon et al., 2010; Aadrean et al., 2011). In their natural habitat, the small-clawed otter prefers to eat crabs rather than other aquatic animals (Kruuk et al., 1994; Hon et al., 2010).
The availability of prey species in a habitat is a main factor influencing prey selection (De Silva, 1991). In addition, geographical factors such as latitude, altitude, and climate determine the eating habits of a species (Remonti et al., 2009). Variations in food resources can influence the selection of animal habitats (Wang et al., 2010). Research on diet composition can provide an overview of animal ecological niches and help to design strategies in conservation management in a landscape (Kruuk, 2006). Furthermore, a landscape has a spatio-temporal dynamic that influences the ecological interaction of the organisms. However, previous studies on small-clawed otter diet composition have not yet revealed information on its relationship to temporal environmental factors (Kruuk et al., 1994; Hon et al., 2010; Foster Turley, 1992; Kanchasanaka and Duplaix, 2011; Aadrean et al., 2011; Abdul-Patah et al., 2014).
The existence of small-clawed otters in the paddy fields of West Sumatra had been reported by Aadrean et al., (2010). Paddy fields are an inundated aquatic ecosystem that supports various types of aquatic animals (Che Salmah et al., 2017). Paddy ecosystems have a high diversity of fauna. This diversity is largely determined by the environmental conditions of the rice fields (Heong et al., 1991). In addition, Aadrean and Usio (2017; 2020) have also described the characteristics of latrine site of small-clawed otters in the West Sumatra paddy field and its relationship to environmental factors. However, the research did not report on diet composition of small-clawed otter, and its relationship to temporal environmental factors. The purpose of this study, therefore, was to determine the effect of environmental factors of paddy on the diet of small- clawed otters.
STUDY SITE AND METHOD
Study site
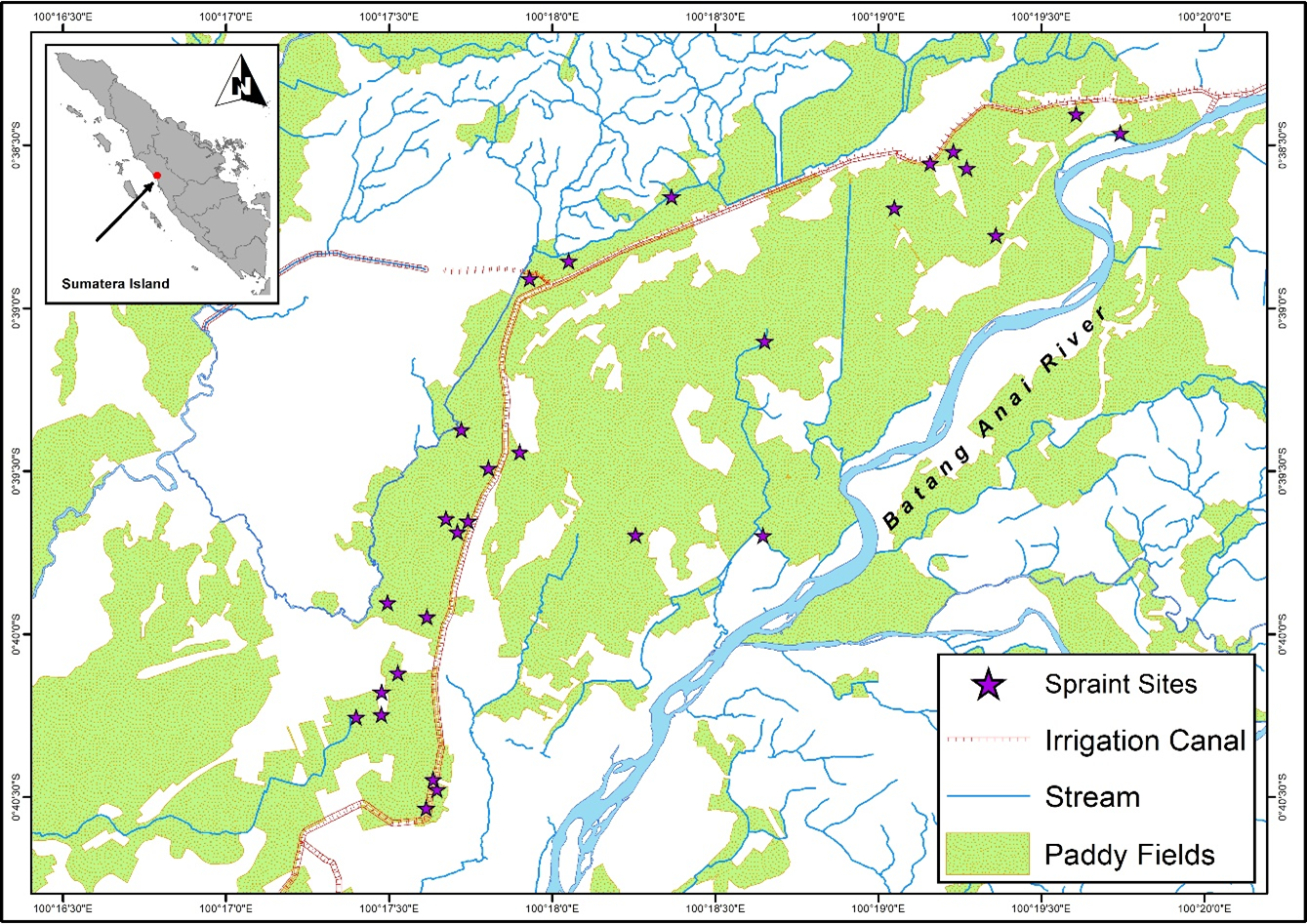
We studied small-clawed otters in paddy fields in Lubuk Alung sub-district, Padang Pariaman Regency, West Sumatra, Indonesia (00°38'00''S – 00°40'40''S, 100°17'10'' – 100°20'20'' E, with altitude 25 – 50 m above sea level) (Fig. 1). The paddy field landscape has an area of 3.139 Ha, with most of them (2.815 Ha) irrigated with technical irrigation from the Anai Dam irrigation project. This area has a tropical climate. Rainfall in Lubuk Alung has a range of 138 – 853.2 mm/month, with an average of 558.225 mm/month (Statistics Indonesia, 2013-2017).
The paddy fields in this study were owned and managed by individuals or small groups of farmers. Paddy cultivation in the study site can take place two or three times per year. Because there is no great fluctuations in climate conditions, and thertr is all-day availability of water from the Anai Dam, farmers may determine for themselves when to plant rice in their paddy fields. As a result, the paddy cultivation stage was asynchronous, creating heterogeneous paddy field landscape.
Otter Diet
We examined 415 spraints collected from the Lubuk Alung paddy field from March 2015 to April 2017. The spraints were collected weekly from 25 latrine sites recorded during a previous study (Aadrean and Usio, 2017), which investigated latrine site characteristics and visitation rates. While collecting each spraint specimen, Aadrean and Usio (2017) recorded rice cultivation stage and water level of the paddy field adjacent to the latrine site. Prior to identification, the spraints were washed in water and then air-dried. For identification, we used reference material created from prey species collected in the study site.
Diet composition was analyzed by occurrence frequency, relative occurrence frequency (Anoop and Hussain, 2005) and Score-Bulk Estimate (SBE) (Fonseca et al., 2008). Then to conclude, we used the Rescaled Important Index (RII) proposed by Fonseca et al., (2008). Scores of SBE were obtained by multiplying the dry weight of the spraint with the visual proportion value (ranged from 1 to 10) of each prey animal category.
To reveal any relationship between temporal environmental factors and diet composition, we performed statistical modeling. We used the SBE of each diet composition category for response variable (Table 1). We only selected prey categories that had an occurrence frequency of more than 10% (fishes, molluscs, insects and frogs). We used cultivation stage, water level of paddy fields, temperature and rainfall as predictor variables. The temperature and rainfall data were obtained from Meteorological, Climatological, and Geophysical Agency (BMKG) data extracted from annual profile of Padang Pariaman regency (Statistics Indonesia, 2016-2018). Cultivation stage wase divided into 4 categories: preparation, vegetative, generative, and postharvest. Preparation stage is the period starting from when paddy fields are ploughed until the farmers plant the rice seedlings. Vegetative stage is the period from farmers starting to plant rice until the emergence of the paddy flowers. Generative stage starts from the period of flowering until farmers harvest the rice. Postharvest is when the period after harvesting until the fields are ploughed again.
We examined the relationship in two levels of model; spraint site level and landscape level. In spraint site level, we used diet composition of each spraint specimen. In landscape level, we pooled diet composition of spraints that were collected from each weekly sampling effort. Thus, we averaged the SBE and other environmental factors, except for cultivation stage, where we used dominant stage as the input data (Table 2).
We explored all combination between predictor variable and then analyzed using the General Linear Model (GLM). The best model is selected based on the value of Akaike’s Information Criterion (AIC). The best modeling is chosen based on the smallest AIC value. When there are two or more models that have the smallest AIC value (ΔAIC <2), the model that has the least number of parameters is chosen.
RESULTS
We found that the diet composition of small-clawed otters in the paddy fields consist of fishes, snails, insects, frogs, crabs, mammals, birds and reptiles. Fish showed as dominant composition in the diet. Remains of fish were almost found in every spraint specimen (99.04% of occurrence frequency). We found snails (dominantly golden-apple snails) in high occurrence frequency (91.08%) in the diet composition, although SBE of the category was not such high (24.84%). Remains of crabs were found in only 4.10% occurrence frequency (Table 3).
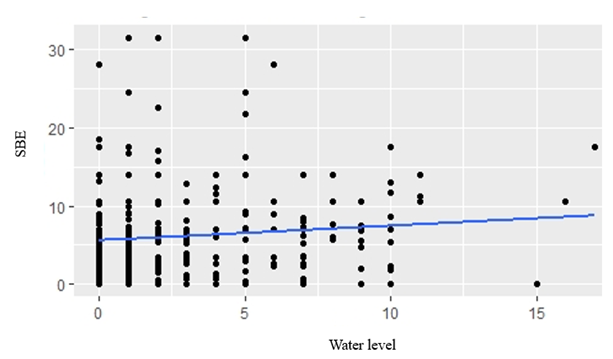
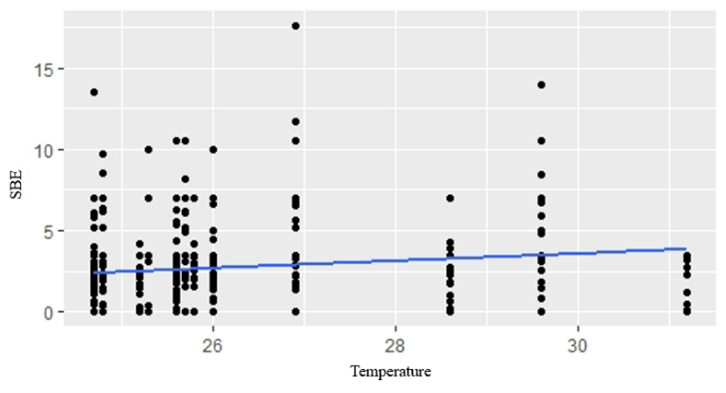
GLM analyses revealed that temperature, rainfall, water level and cultivation stage were not significantly related to fish composition in small-clawed otter diet (Table 4). The best model for fishes in both spraint-site and landscape level was the model without predictor variable (null model) (Table 5). Water level showed positive relationship to snails in the diet composition in spraint site level (AIC = 2508.53, P=0.0449) (Fig. 2). However, there was no predictor has significant relationship in landscape level. Temperature showed positive relationship to insect and frogs in the diet composition in both spraint site and landscape level (all P<0.01) (Fig. 3; Fig. 4).
 Spraint Site Level
Spraint Site Level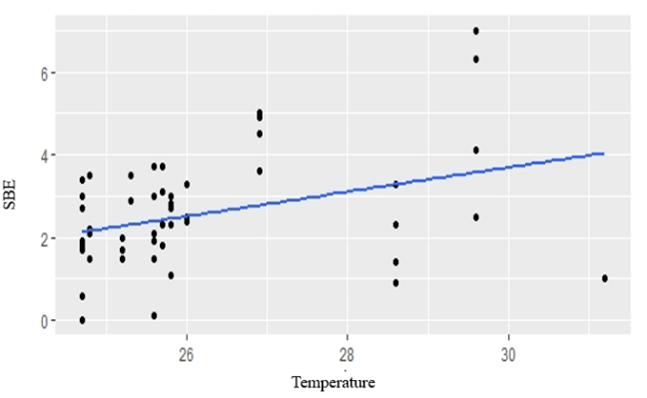 Landscape Level
Landscape Level
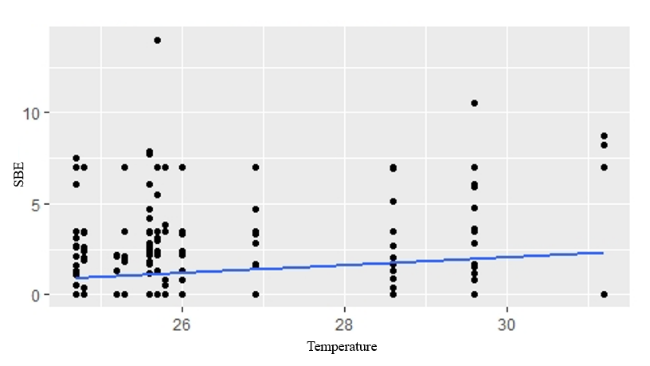 Spraint Site Level
Spraint Site Level
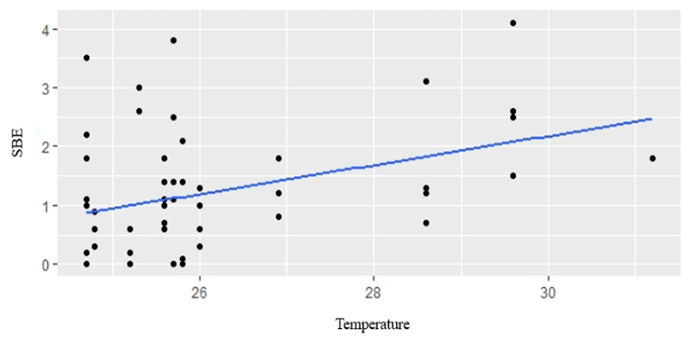 Landscape Level
Landscape Level
DISCUSSION
Diet Composition
This study found that fish are the dominant diet of small-clawed otter. This result differs from studies in other natural habitats, such as in Thai rivers (Kruuk et al., 1994), in the northeastern rivers of Cambodia (Hon et al., 2010), in peat swamp forests of southern Thailand (Kanchanasaka and Duplaix, 2011) that showed crabs as a dominant food, and in combined habitat type in peninsular Malaysia that showed insects as a dominant 0Abdul-Patah et al., 2014) (Table 6). Habitat changes may cause the transition of dominant prey. Because otters are opportunistic predators, they will forage on prey species according to the prey available in their habitat (Clavero et al., 2003).
The availability of prey is the main factor that determines the diet of otters in their habitat (De Silva, 1991; Foster-Turley, 1992; Kruuk, 2006). Crab is lacking in our study site. As a comparison, paddy fields in Java that were described by Melisch et al. (1996) were destroyed by Potamon crabs. Paddy fields with irrigation canals allow fishes to be available as prey to small-clawed otters. The existence of paddy fields that are close to community settlements that have fish ponds, can be also a factor that causes fish to become the dominant food, although it is necessary to examine whether the small-clawed otters in this location prey on fish from community ponds or from small watercourses in the paddy fields.
The snail category also appeared as dominant in the diet composition of small clawed otters (91.08% occurrence frequency). Golden apple snail (Pomacea canaliculata) is abundant in this paddy field. We could not confirm yet whether the snails are food substitution for crabs or not; however, the morphology of the last two, large, upper teeth of small-clawed otters are specialized to crush crabs and other hard-shelled prey (Hussain et al., 2011). The Golden apple snail is an invasive species that has developed into a pest of rice plants in rice fields throughout the world (Suharto, 2002). Golden apple snails are therefore classified as the 100 worst invasive species in the world by the Invasive Species Group (ISSG) (Lowe et al., 2000). This result may be used to design the future development of environmentally friendly agriculture. Without using chemicals, farmers may control the golden apple snail pest by using the small-clawed otters as natural pest controls. Further research is necessary to confirm the effectiveness of this.
The Relationship of Environmental Factors with the Diet of Small-Clawed Otters.
Interestingly, there was no predictor for a relationship with fishes in the diet composition of small-clawed otter. This finding suggests that, availability of fishes in the paddy field did not relate to the dtage of cultivation. Cultivation in our study site is asynchronous. Even though one paddy field has entered a post-harvesting stage, the other paddy fields may still in vegetative stage. In such conditions, the canals and ditches are mostly inundated by water throughout the year. The main source of fishes is probably the irrigation ditches and canals. Moreover, in tropical climate regions, the growth and reproduction cycles of fishe are mostly asynchronous. The availability of fish will be biologically stable throughout the year.
Snails in the diet of small-clawed otters had a positive correlation with water level. The slow moving traits of snails limit them to be highly depend on the conditions in a particular paddy field. According to a previous study (Aadrean and Usio, 2020), the availability of snails was greatest when the water level was higher. Furthermore, Widjajanti (1989) revealed that the snail population tends to decrease in the last third of the period of rice growth and before the harvest, because at that time the paddy water is reduced or almost dry.
The composition of insects in the diet is positively related to temperature. This is in accordance with the statement of Haneda et al., (2013) that an increase in temperature will affect the activity of insects, distribution, and breeding. Higher temperature will increase availability of insects in the paddy field. Similarly, the amountof frogs in otter diets is also positively related to temperature. The presence of animals that are exothermic, such as insects and amphibians, is closely correlated to environmental temperature. In the study of Browne and Edward (2003), temperature influences the growth and development of an amphibian species. Amphibians are an important part of the otter diet in many locations (Erlinge, 1972; Brzeziñski et al., 1993; Sulkava, 1996). Most of the research conducted to look at the annual pattern of the highest amphibian consumed by otters occurs in late winter and early spring (Weber, 1990; Brzeziñski et al., 1993; Sulkava, 1996).
Our study results may be used to enhance paddy cultivation management. If we consider an environmentally friendly agriculture system, water management and asynchronous cultivation system should be included in the consideration.
Acknowledgements: We would like to thank Mahfud Huda, Evan Ananta, Team Verteb Andalas University and all members of Museum Zoology Andalas University. This study was funded by Andalas University (contract number T/49/UN.16.17/PT.01.03-IS-RD/2020). The spraint collection project was funded by Rufford Small Grants (No.17544-2).
REFERENCES
Aadrean, Usio, N. (2017). Small-clawed otters (Aonyx cinereus) in Indonesian rice fields: latrine site characteristics and visitation frequency. Ecological Research, 32: 899-908
Aadrean, Usio, N. (2020). Spatiotemporal patterns of latrine-site use by small-clawed otters in a heterogeneous rice field landscape. Mammal Study 45: 103–110
Aadrean, Novarino, W., Jabang. (2011). A record of small-clawed otters (Aonyx cinereus) foraging on an invasive pest species, golden apple snails (Pomacea canaliculata) in a West Sumatra rice field. IUCN Otter Specialist Group Bulletin, 28: 34-38.
Aadrean, Salmah, S., Salsabila, A., Rizaldi., Janra, M. N. (2010). Tracks and other signs of otters in rice fields in Padang Pariaman, West Sumatra: a preliminary study. IUCN Otter Specialist Group Bulletin, 27: 6-11.
Abdul-Patah, P., Nur-Syuhada, N., Md-Nor, S., Sasaki, H., Md-Zain, B.M. (2014). Habitat and food resources of otters (mustelidae) in peninsular malaysia. AIP Conference Proceedings 1614: 693–699. DOI: https://aip.scitation.org/doi/abs/10.1063/1.4895286
Anoop, K.R., Hussain, S. A. (2005). Food and feeding habits of smooth-coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology, 266:15-23.
Browne, R.K., Edwards, D.L., (2003). The effect of temperature on the growth and development of the endangered green and golden bell frog (Litoria aurea). J. Therm. Biol. 28: 295–299.
Brzeziñski, M., Jedrzejewski W. and Jedrzejewska B. (1993). Diet of otters (Lutra lutra) inhabiting small rivers in the Bialowieza National Park, eastern Poland. Journal of Zoology, 230: 495-501.
Che Salmah, M.R., Siregar, A.Z., Abu Hassan, A., Nasution, Z. (2017). Dynamics of aquatic organisms in a rice field ecosystem: effects of seasons and cultivation phases on abundance and predator-prey interactions. Tropical Ecology 58: 177-191
Clavero, M., Prenda, J., Delibes, M. (2003). Trophic diversity of the otter (Lutra lutra). in temperate and Mediterranean freshwater habitats. Journal of Biogeography. 30: 761-769.
De Silva, P.K. (1991). Distribution of Lutra lutra in the Highlands of Sri Lanka IUCN Otter Spec. Group Bull. 6: 2-5
Erlinge, S. (1972). Interspecific relations between otter (Lutra lutra) and mink (Mustela vison) in Sweden. Oikos, 23: 327-335
Fonseca, V.C., Rheingantz, M.L., Fernandez, F.A. (2008). A comparison of two different methods for estimating the diet of the neotropical otter, Lontra longicaudis, with the proposal of a new index for dietary studies. IUCN Otter Spec. Group Bull. 25: 6-12
Foster-Turley, P. (1992). Conservation aspects of the ecology of Asian small-clawed and smooth otters on Malay peninsula. IUCN Otter Spec. Group Bull. 7: 26 -29
Haneda, N.F., Kusmana, C., Kusuma, F.D. (2013). Insect diversity in mangrove ecosystems. Jurnal Silvikultur Tropika. 4: 42-46.
Heong, K.L., Aquino, G.B., Barrion, A.T. (1991). Arthropod community structures of rice ecosystems in the Philippines. Bulletin of Entomological Research, 81: 407-416.
Hon, N., Neak, P., Khov, V., Cheat, V. (2010). Food and habitat of Asian small-clawed otter in northeastern Cambodia. IUCN Otter Spec Group Bull 27: 12-23.
Hussain, S.A., Gupta, S.K., De Silva P.K. (2011). Biology and ecology of Asian small-clawed otter Aonyx cinereus (Illiger, 1815): a review. IUCN Otter Spec Group Bull 28: 63–75.
Kanchasanaka, B., Duplaix, N. (2011). Food Habits of the Hairy-nosed otter (Lutra sumatrana) and the Small-clawed otter (Aonyx cinereus) in Pru Toa Daeng Peat Swamp Forest, Southern Thailand. IUCN Otter Spec. Group Bull. 28(A): 139-149.
Kruuk, H. (2006). Otters: Ecology, Behaviour and Conservation. Oxford University Press. ISBN-13: 9780198565871. DOI:10.1093/acprof:oso/9780198565871.001.0001
Kruuk, H., Kanchanasaka, B., O'Sullivan, S., Wanghongsa, S. (1994). Niche separation in three sympatric otters Lutra perspicillata, L. lutra and Aonyx cinereus in Huai Kha Khaeng, Thailand. Biol. Conserv. 69: 115-120.
Lowe, S., Browne, M., Boudjelas, S., De Poorter, M. (2000). 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. IUCN Invasive Species Specialist Group (ISSG).
Melisch, R., Asmoro, P.B., dan Kusumawardhami, L. (1994). Major Steps Taken Towards Otter Conservation in Indonesia. IUCN Otter Spec. Group Bull. 10: 21-24.
Melisch, R., Kusumawardhani, L., Asmoro, P.B., Lubis, I.R., (1996). The Otters of West Java: A Survey of Their Distribution and Habitat Use and a Strategy Towards a Species Conservation Programme. PHPA/Wetlands International - Indonesia Programme, Bogor.
Okamoto, Y., Maeda, N., Hirabayashi, M., Ichinohe, N., (2020). The Situation of Pet Otters in Japan – Warning By Vets. IUCN Otter Spec. Group Bull. 37 (1):71 – 79
Remonti, L., Balestrieri, A., Prigioni, C. (2009). Altitudinal gradient of Eurasian otter (Lutra lutra) food niche in Mediterranean habitats. Canadian Journal of Zoology, 87(4): 285–291.
Sivasothi, N., Nor, B.H.M. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia 285: 151-170.
Suharto, H. (2002). Golden Apple Snail, Pomacea canaliculata (Lamarck) in Indonesia. In: Wada, T., Yusa, Y., Joshi, R.C., Ghesquiere, S. (eds.). Proceedings of the special working group on the golden apple snail (Pomacea spp.). The Seventh International Congress on Medical and Applied Malacology (7th ICMAM).
Sulkava, R. (1996). Diet of otters Lutra lutra in central Finland. Acta Theriologica, 41: 395-408.
Wang, J., Gao, W., Wang, L., Metzner, W., Ma, J., Feng, J., (2010). Seasonal variation in prey abundance influences habitat use by greater horseshoe bats (Rhinolophus ferrumequinum) in a temperate deciduous forest. Can. J. Zool. 88: 315-323.
Weber, J. (1990). Seasonal exploitation of amphibians by otters (Lutra lutra) in north-east Scotland. Journal of Zoology, 220: 641-651.
Widjajanti, S. (1989). Studies on the Biology of Lymnaea rubiginosa. MSc Thesis. James Cook University of North Queensland, Townsville, Queensland, Australia.
Wright, L., de Silva, P., Chan, B., Reza Lubis, I. (2015). Aonyx cinereus. The IUCN Red List of Threatened Species 2015: eT44166A21939068. https://dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T44166A21 939068.en.
Resumé: Relation entre Facteurs Environnementaux Temporels et Composition du Régime Alimentaire de la Loutre Cendrée (Aonyx cinereus) dans un Paysage de Rizières Hétérogène à Sumatra, en Indonésie
La loutre cendrée (Aonyx cinereus) est l'une des quatre espèces de loutres en Indonésie. Cette espèce consomme différents espèces d'animaux. La disponibilité en proie dépend de ses conditions environnementales. En conséquence, cette étude visait à examiner la relation entre les facteurs environnementaux temporels et la composition du régime alimentaire de la loutre cendrée dans un paysage des rizières. Nous avons examiné 415 épreintes collectées dans ce paysage hétérogène de rizières à l'ouest de Sumatra, en Indonésie. Nous avons constaté que la loutre cendrée consommait des poissons, des escargots, des crabes, des reptiles, des grenouilles, des oiseaux et des mammifères. Contrairement au milieu naturel, le poisson est devenu le régime alimentaire dominant plutôt que les crabes. De plus, nous avons effectué une analyse à l’aide du Modèle Linéaire Généralisé (MLG) pour expliquer les facteurs environnementaux temporels qui affectent la composition du régime alimentaire de la loutre cendrée. Les analyses du MLG ont révélé que la température, les précipitations, le niveau de l'eau et le stade de la culture n'étaient pas significativement corrélés à la composition des poissons du régime alimentaire de la loutre cendrée. Il y avait une corrélation positive entre le niveau de l'eau et la présence d’escargots dans la composition du régime alimentaire. De même, il existait une corrélation positive entre la température et la présence d’insectes et de grenouilles dans la composition du régime alimentaire.
Revenez au dessus
Resumen: Relación entre Factores Ambientales Temporarios y Composición de la Dieta en la Nutria de Uñas Pequeñas Asiática (Aonyx cinereus) en el Paisaje Heterogéneo de Arrozales en Sumatra, Indonesia
La nutria de uñas pequeñas asiática (Aonyx cinereus) es una de las cuatro especies de nutria que hay en Indonesia. Esta especie consume varios tipos de animales. La disponibilidad de presas depende de las condiciones ambientales. Por lo tanto, este estudio estuvo dirigido a examinar la relación entre factores ambientales temporarios y la composición de la dieta de la nutria de uñas pequeñas, en un paisaje de arrozales. Examinammos 415 fecas colectadas en un paisaje heterogéneo de arrozales en Sumatra Occidental, Indonesia. Encontramos que la nutria de uñas pequeñas consume peces, caracoles, cangrejos, reptiles, ranas, aves, y mamíferos. A diferencia de los hábitats naturales, los peces fueron dominantes en la dieta, en lugar de los cangrejos. Además, llevamos a cabo un análisis de Modelo Lineal Generalizado (GLM) para explicar los factores ambientales temporarios que afectan la composición de la dieta de la nutria de uñas pequeñas. Los análisis GLM revelaron que la temperatura, precipitación, nivel del agua y estadío de cultivo no estuvieron relacionados significativamente con el peso de los peces en la dieta de las nutrias. El nivel del agua mostró una relación positiva con el peso de los caracoles en la composición de la dieta. La temperatura mostró una relación positiva con el peso de los insectos, y de las ranas, en la composición de la dieta. showed positive relationship to both insect and frogs in the diet composition.
Vuelva a la tapa