IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 40 Issue 1 (January 2023)
Citation: Haring, C., Weier, S. and Linden, B. (2023). Distribution and Habitat Preference of Cape Clawless Otters (Aonyx capensis) and Water Mongooses (Atilax paludinosus) in the Soutpansberg, South Africa. IUCN Otter Spec. Group Bull. 40 (1): 26 - 38
Distribution and Habitat Preference of Cape Clawless Otters (Aonyx capensis) and Water Mongooses (Atilax paludinosus) in the Soutpansberg, South Africa
Céline Haring1,Sina Weier2, and Birthe Linden2, 3*
1HAS University of Applied Sciences, Onderwijsboulevard 221, 5223 DE ’s-Hertogenbosch, The Netherlands
2SARChI Chair on Biodiversity Value & Change, Faculty of Science, Engineering and Agriculture, University of Venda, Private Bag X5050, Thohoyandou, 0950 South Africa
3Lajuma Research Centre, P.O. Box 522, 0920 Louis Trichardt, South Africa
*Corresponding Author Email: bibi@lajuma.com
Received 17th September 2022, accepted 4th October 2022
Abstract: Over 84% of the river ecosystems in South Africa are threatened and, accordingly freshwater dependent species such as the Cape clawless otter (Aonyx capensis) and the water mongoose (Atilax paludinosus) are also declining in numbers. These species share a similar diet and habitat preference and in certain places in South Africa it is known that they occur in sympatry. Our study focused on a pristine river system in the far western Soutpansberg where little is known about the local distribution and habitat preferences of these species. To determine the distribution and fine scale habitat preferences of otters and water mongooses, tracks and signs (TS) and camera traps were used, and spraint content analysed to establish differences in diet. Based on the TS that were found, the Cape clawless otter and water mongoose are both widely distributed along the river system and mostly occur separate from each other. The observed amount of TS of Cape clawless otters was higher in areas with pools, rocky riverbanks and areas with a stream width of 2 - >5 m in diameter. The number of water mongoose TS recorded was higher in wetland areas with leafy riverbanks and areas with a stream width of up to 2 m. We suggest that Cape clawless otters and water mongooses may avoid direct competition by habitat partitioning in the western Soutpansberg..
Keywords: Camera traps, mountain range, Limpopo Province, spraints, niche separation
INTRODUCTION
Freshwater ecosystems, which play a vital role in sustaining life, are one of the most endangered habitats on the planet (Harrison et al., 2010; Green et al., 2015). Alien species, overexploitation, modification of waterflow, water pollution and transformation, global climate change and loss of habitat are the main threats to freshwater ecosystems (Allan and Flecker, 1993; Malmqvist and Rundle, 2002; Rahel, 2002; Dudgeon et al., 2005; Revenga et al., 2005), with biodiversity loss in these systems appearing to be even greater than recorded for any of the most affected terrestrial ecosystems (Sala et al., 2000).
In South Africa, over 84% of the river ecosystems are threatened (Nel and Somers, 2007) and with this many terrestrial species that are water dependent (Revenga and Kura, 2003; Balian et al., 2008), including the Cape clawless otter (Aonyx capensis), spotted-necked otter (Hydrictis maculicollis) and water mongoose (Atilax paludinosus). According to the IUCN, Cape clawless otter and spotted-necked otter populations are declining, resulting in the two otter species globally listed as Near Threatened (Jacques et al., 2021; Reed-Smith et al., 2021). On a regional scale, the Cape clawless otter is also listed as Near Threatened (Okes et al., 2016) whereas the spotted-necked otter is listed as Vulnerable (Ponsonby et al., 2016). The water mongoose is listed as Least Concern both globally and regionally, with global population trends decreasing and regional population trends unknown (Do Linh San et al., 2015; Baker et al., 2016). In certain localities in South Africa it is known that Cape clawless otter, spotted-necked otter and water mongoose occur sympatrically, suggesting that these three species share similar habitat and diet preferences (Skinner and Smithers, 1990; Rowe-Rowe, 1991).
The Soutpansberg mountain range in far northern South Africa is a nationally recognised Strategic Water Source Area (SWSA; Le Maitre et al., 2018) with numerous perennial streams and rivers providing suitable habitat for otters and water mongooses. While there is substantial anthropogenic pressure on freshwater ecosystems in the eastern parts of the mountain range (Le Maitre et al., 2018), the western Soutpansberg is comparatively intact due to the low human population density (Statistics South Africa, 2012).
Our study focused on a pristine river system in the far western Soutpansberg where Cape clawless otters and water mongooses are known to occur (Baker et al., 2016; Okes et al., 2016; GBIF.org, 2022 a,b), but where spotted-necked otters have not been recorded. Little is known about the local distribution and habitat preferences of these species in far northern South Africa. Previous studies on the three species suggest some degree of niche separation along river systems between otters and mongooses, with the former expected to prefer shallow to deep pools with rocky riverbanks and dense vegetation and the latter preferring wetland areas with dense vegetation (Rowe-Rowe, 1977; Stuart, 1981; Kingdon, 1997; Perrin and Carugati, 2000; Nel and Somers, 2007; Kundu et al., 2008). In this study we aim to add more detailed spatial and ecological data from this so far understudied area of the species’ national distribution range through a first systematic survey.
MATERIALS AND METHODS
Study area
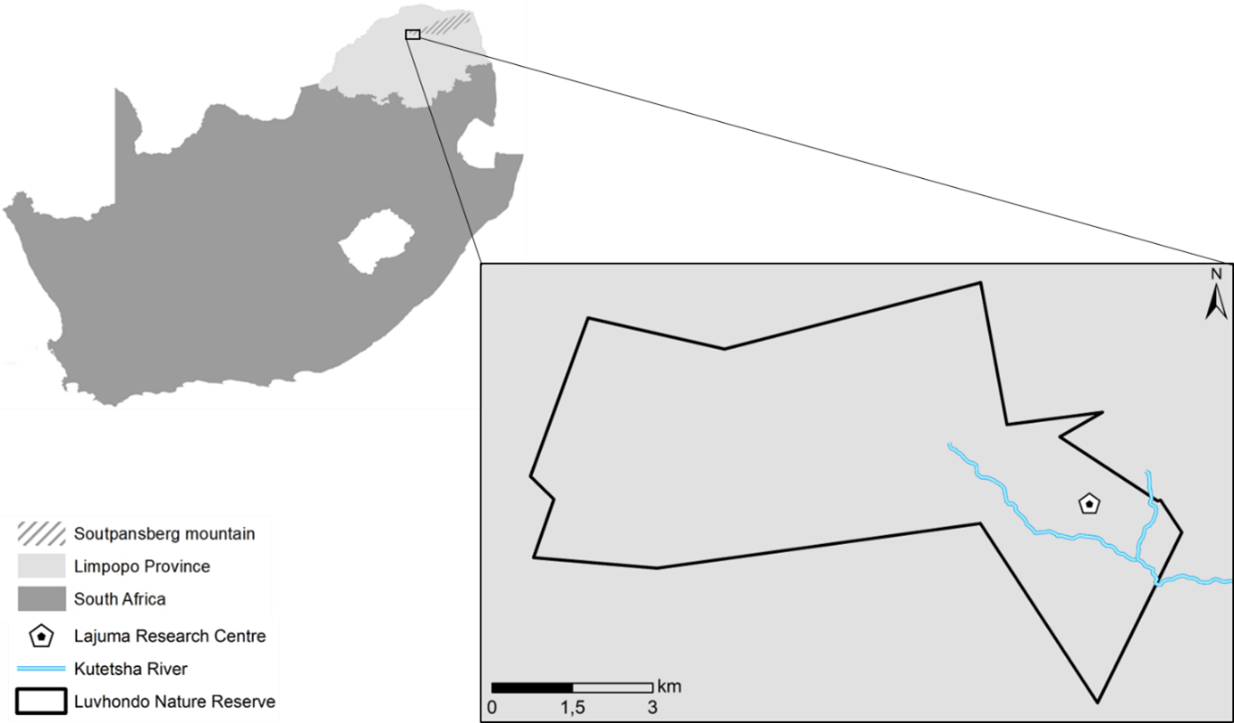
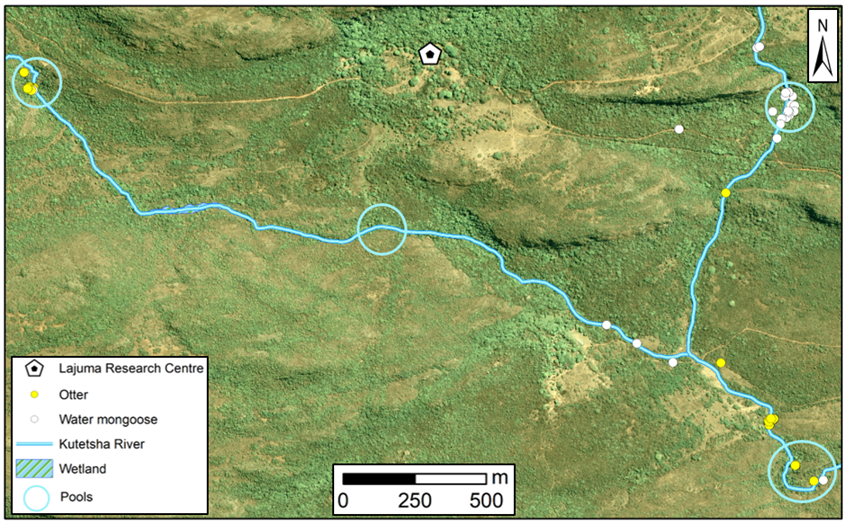
The study was conducted at the Lajuma Research Centre, a private property situated on the southern slopes of the western Soutpansberg (23°02'17.1"S 29°26'26.5"E; elevation: 1000-1300 masl). It is part of the Luvhondo Nature Reserve, which lies within the UNESCO Vhembe Biosphere Reserve. Our study focused on one of the main north-south flowing rivers in the reserve, the Kutetsha and one of its tributaries (Fig. 1). The banks surrounding the Kutetsha and its tributary range from rocks, to gallery forest and marshy wetland areas and natural pools are formed regularly along the course of the system. Riparian vegetation along this river system generally consists of tall grass, reed beds and trees (Fig. 2). The Kutetsha river system is a tributary to the Hout river, in turn a tributary of the Sand river within the Limpopo river catchment area.

Data collection
Tracks and signs
To establish occurrence and habitat preference of the three species, we conducted surveys for tracks and signs (TS) over a 10-week period between February 2019 and June 2019. Surveys consisted of slowly walking along and visually scanning both banks of the Kutetsha river and its tributary to search for TS: feeding sites and spraints using a tracks and signs field guide for identification (Stuart and Stuart, 2013). In total, five km of river were surveyed during 48 survey walks. Three starting points were chosen for the TS survey, moving downstream from north to south. Each section of the river was surveyed 16 times during the 10-week survey. GPS coordinates were taken for all TS found.
River characteristics
To determine habitat preference by species, a description of the environment was recorded at the location at which TS were found. Four river characteristics were described: location (river, pool, wetland), substrate (leaf litter, rocks, silts), visual estimate of stream width (< 0.5 m, 0.5-1 m, 1-2 m, 2-5 m, > 5 m or ‘not applicable’) and water depth (< 0.5 m, 0.5-1 m, 1-2 m, > 2 m or ‘not applicable’). The criteria ‘not applicable’ was used whenever TS were found away from the river system. Stream width and depth classes were recorded by either wading into the river and estimating the depth or by visual estimates in areas where the river was too deep for wading in (Harding et al., 2009).
Spraint content
Fresh spraints were collected for dietary content analysis. Spraints were collected in a paper bag, dried in an outdoor environment and stored until analysis. The spraint content was analysed using a magnifying glass with a magnification of 2.5x. The content of the spraints was categorised as crustaceans, small mammals and insects (Jacobsen, 2004; Kloskowski, 2005; Palazón et al., 2008; Guertin et al., 2010).
Camera trap survey
Wherever TS were found, a camera trap (Ltl Acorn LTL-6210MC HD Trail Camera) was deployed in the area to visually confirm presence of the species and left for a duration of minimum seven days. The camera settings were set on “picture” (12MP), at which 3 images were taken after movement was detected by the camera. An interval of one second was set between each picture. When pictures of an otter species or water mongoose were recorded, the camera was left in the location until no new pictures of the species were recorded. If after seven days no otters or water mongooses were recorded, the camera trap was moved to another locality where TS were found.
Data analysis
Given that our data collection only included presence data, we chose descriptive data exploration over statistical analyses. Maps were created using ArcGIS 10.5.
RESULTS
Distribution
No TS or camera trap images of spotted-necked otters were recorded, hence all analysis focussed on water mongooses and Cape clawless otters. Based on the TS that were found, Cape clawless otter and water mongoose both occurred along the river system sections surveyed in this study (Fig. 3). The TS that were found suggests that both species seem to largely occur separately from each other. Only one area along the river system was found where both species overlapped. Five Cape clawless otter TS were recorded ad libitum on a single lane dirt road in the Nature Reserve, 317 m away from the river system surveyed (Fig. 3). These outlying distribution records were excluded from further analysis.
Habitat preference
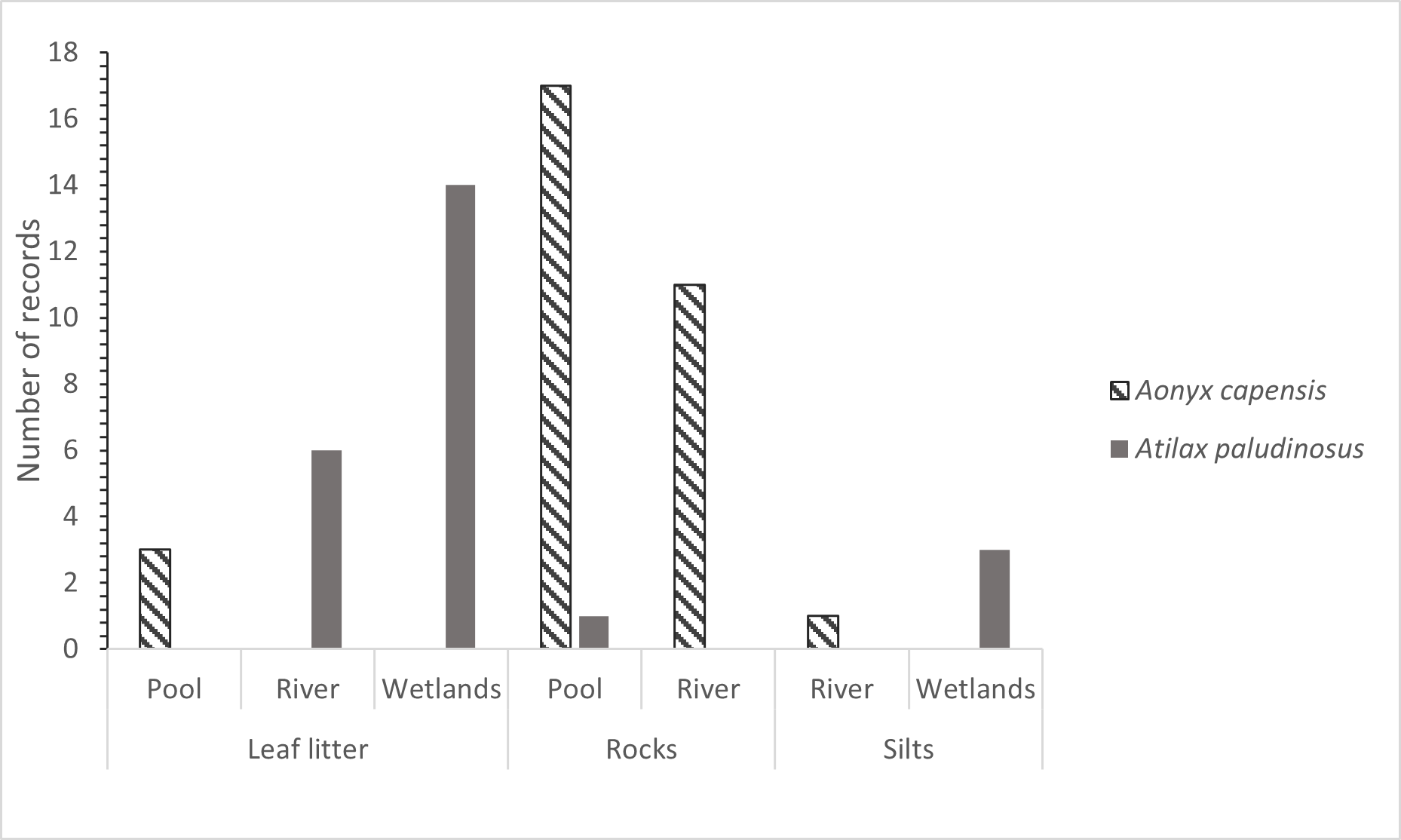
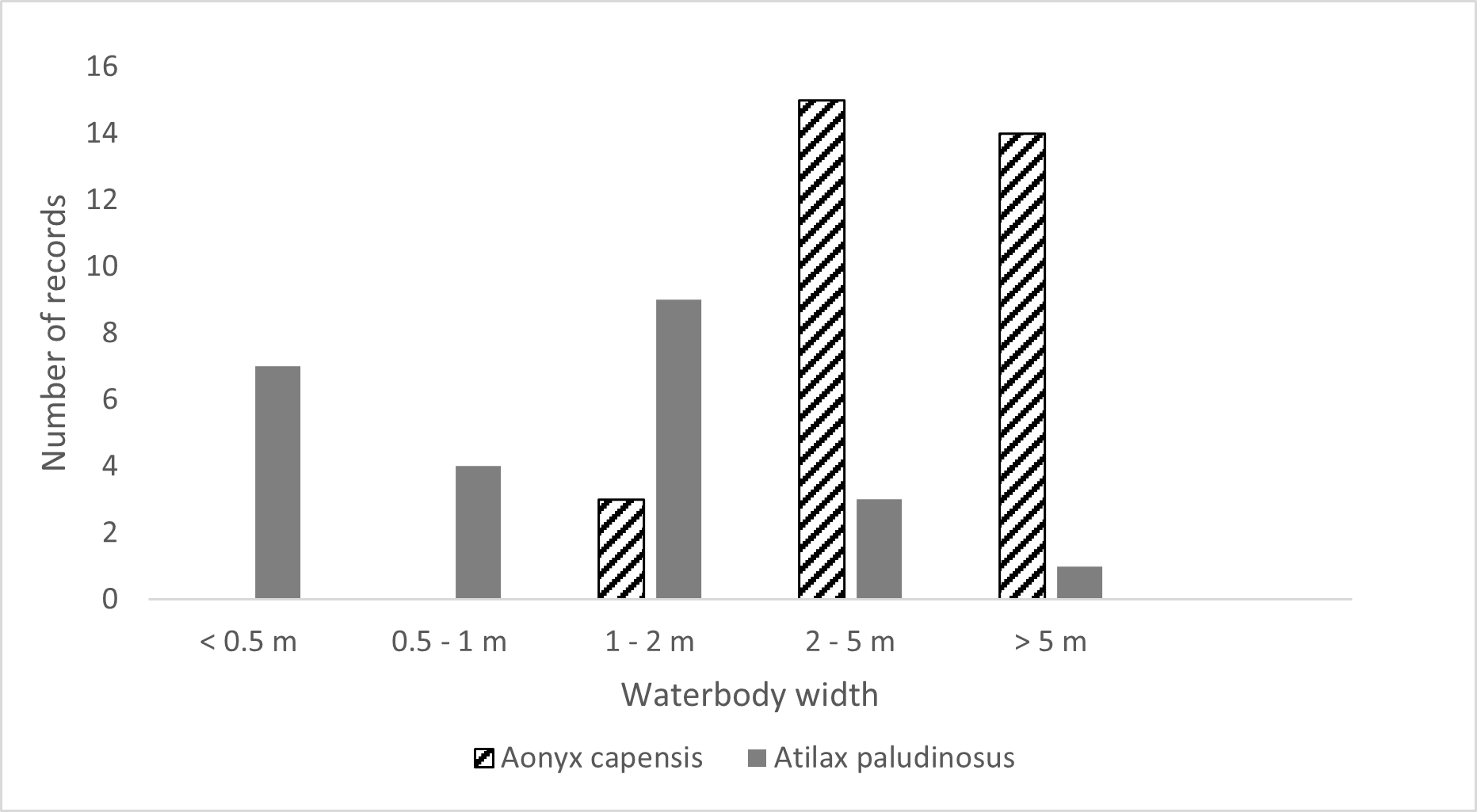
We collected a total of 32 Cape clawless otter TS of which most were found at pools (63%) and on rocky substrate (88%) (Fig. 4). Only one Cape clawless otter TS was found on silts and no TS were found in wetland areas (Fig. 4). In comparison, we found the majority of the 24 collected water mongoose TS on the substrate type leaf litter (83%) and in wetlands (71%) with only one TS found on rocks (Fig. 4). While the two species did not seem to have a preference for a certain water depth (Table 1), we found more TS of the Cape clawless otter at wider areas of the river of 2 to >5 m (91%) while we found more TS of water mongooses at locations with a narrow waterbody of up to 2 m (88%) (Fig. 5).
We collected one fresh water mongoose spraint and four fresh Cape clawless otter spraints. The spraint of the water mongoose was collected near a waterfall. The content of the latter consisted exclusively of crustaceans, including abdomina, legs and several halves of pincers. All spraints of Cape clawless otters were collected near a waterfall and a pool. All otter spraints collected also exclusively consisted of crustaceans, with the carapace, abdomina, legs, whole and halve pincers found.
Camera Trap Survey
Cameras were deployed in five different localities and recorded 3622 pictures including 16 images of otters and two of water mongooses (Fig. 6). Camera trap localities included two areas with natural pools (below a waterfall), one shallow-river area surrounded by gallery forest and marshy wetland, one rocky shallow-river area lined by shrubs and low canopy trees, and one area with gallery forest and natural pools. In one of the five survey localities (area with natural pools, situated below a waterfall) camera traps captured images of both Cape clawless otters and water mongoosesImages of the Cape clawless otter were taken throughout the day with most images (n=9) taken at night-time, between 18:00 and 00:00. The two images of the water mongoose were also taken between 18:00 and 00:00.

DISCUSSION
Distribution
We found that both Cape clawless otters and water mongooses occurred along the Kutetsha river system sections surveyed in our study. Results from the TS suggest that both species mostly occur spatially separate from each other. However, camera trapping revealed that both species can occur in the same locality (area with pools and a waterfall) despite no water mongoose TS found in the area during our surveys. Rowe-Rowe (1991) stated that the Cape clawless otter and water mongoose coexist in several areas in Africa and Skinner and Smithers (1990) stated that the two species use similar habitats. A longer-term survey using camera traps in our study area would be necessary to reach more conclusive results. The presence of the spotted-necked otter could not be confirmed in this study.
Habitat Preference
Cape Clawless Otter
Differences were clearly observed for all habitat variables investigated, except for water depth. TS of Cape clawless otters were more frequently recorded in areas with a rocky substrate and areas where the river was wide (e.g. pools). It was expected that the Cape clawless otter would occur in areas with shallow pools and rocky banks (Perrin and Curagati, 2000). An explanation for TS of Cape clawless otters found more frequently in localities with pools may be that these pools provide a permanent supply of prey throughout the year (Perrin and Carugati, 2000). Signs of Cape clawless otter presence were more often recorded near shallow water, which could be explained by the foraging behaviour of the Cape clawless otter (Curagati, 1995; Perrin and Curagati, 2000), catching freshwater crabs and frogs in shallow waters (Rowe-Rowe, 1977). Perrin and Curagati (2000) also observed that Cape clawless otter signs were associated with shallow water and pools less than 0.6 m deep. Previous studies found that Cape clawless otters occur in areas with rocky riverbanks where rocks are covered with vegetation, likely as the probability of a high local food biomass is greater (Perrin and Curagati, 2000; Nel and Somers, 2007). Comparable results have been observed in numerous other habitats and studies (Van der Zee 1982; Verwoerd, 1987, Rowe-Rowe, 1992; Butler and Du Toit, 1994). The reason for Cape clawless otter TS found at greater river width in our study might be that most (63%; Table 1) TS were found near pools. According to Stuart (1981) Cape clawless otters are found at a variety of water bodies, such as major river systems but also at small reservoirs and non-perennial streams, which suggest that this species tolerates various waterbody widths. However, this has so far not been studied extensively.
We found several signs of Cape clawless otters on the road, a distance (317 m) away from the river. Cape clawless otters have been found to travel between different water bodies, using existing trails such as roads or game trails (Rowe-Rowe, 1978, 1985) and individuals may travel >30km long distances through sandy, waterless habitat and cross saddles between mountain watersheds (Nel and Somers, 2007).
Water Mongoose
The TS of water mongooses were observed more frequently in wetland areas on leaf litter or silt substrate and in narrow areas of the river system. Although we did not find any differences for the various water depth categories, TS of water mongooses were more frequently recorded (88%) in areas with a water depth of up to 2 m, suggesting a preference for shallow waters. Wetland riverbanks in our study area consisted only of silts and leaf litter. Kundu et al. (2008) categorised the water mongoose as a species which is endemic to wetland areas with leafy banks and shallow waters. The observed TS near shallow water (<0.5 m) could be explained by the water mongoose’s foraging behaviour as they mainly forage in the littoral zones of water bodies (Baker, 1989) generally only submersing their head in the water (Rowe-Rowe, 1977).
Spraint Content
Spraint content analysed for both species consisted exclusively of crustaceans. For Cape clawless otters, this would be expected as they are physically adapted to primarily feed on crustaceans (Rowe-Rowe and Somers, 1998). Previous studies showed that apart from their predominantly freshwater crab based diet, the spraints of Cape clawless otters may also contain frog and insect remains, depending on local prey availability (Turnbull-Kemp, 1960; Donnelly and Grobler, 1976; Rowe-Rowe, 1977; Kruuk and Goudswaard, 1990; Butler, 1994; Ligthart et al., 1994; Purves et al., 1994; Somers and Purves, 1996; Rowe-Rowe and Somers, 1998). Water mongooses are highly adaptable predators with spraints commonly containing aquatic prey items including insects, freshwater crabs and frogs, and terrestrial prey items such as mammals (Somers and Purves, 1996; Rowe-Rowe and Somers, 1998).
Generally, spraints analysed in highland elevations show a high percentage of crabs compared to fish as freshwater crabs are more abundant, and as freshwater prey diversity is overall lower in such localities (Rowe-Rowe, 1977). Fish diversity of the Kutetsha sections surveyed in our study is, with two recorded species (African longfin eel (Anguilla mossambica) and stargazer mountain catfish (Amphilius uranoscopus)), comparatively low (Ian Gaigher, pers. comm.). Regarding frogs, five species have been recorded from the Kutetsha system in the study area (striped stream frog (Strongylopus fasciatus), clicking stream frog (S. grayii), Delalande's river frog (Amietia delalandii), painted reed frog (Hyperolius marmoratus), African clawed frog (Xenopus laevis)) (Jabu Linden, pers. comm.). In addition to elevation, the percentage of other food items included in the diet may also vary depending on differing availability along the river system and over seasons (Rowe-Rowe and Somers, 1998). Lastly, given the very small sample size of spraints analysed in our study, dietary diversity, particularly of water mongooses, may increase with study period and sample size.
Camera Trap Survey
During our survey we found one locality in which camera traps captured both Cape clawless otters and water mongooses. Based on these images it is confirmed that both species may share or overlap in certain areas along the Kutetsha river system. We found that both species were most active in the evening. This was in line with previous studies showing that Cape clawless otters are described to be predominantly active during early morning, late afternoon and early evening in freshwater habitats (Rowe-Rowe, 1978; Maddock and Perrin, 1993). Water mongooses are described to be active in the late afternoon, night and early morning (Maddock and Perrin, 1993).
CONCLUSION
Our study shows that Cape clawless otters and water mongooses have slightly different habitat preferences along river systems in the western Soutpansberg and that there is dietary overlap. Direct food competition between the two species may be avoided since there is possibly enough difference between their diet and/or as food availability is sufficient enough to sustain both species. We recommend that future studies include additional, more detailed habitat variables such as the type of riverbank (rocks, rocks with vegetation, reeds or grass), percentage of vegetation cover on rocks, type of vegetation cover (low grass, high grass or bush) surrounding the water body, and the substrate of the riverbed. Furthermore, we recommend that further research be conducted on both species not only in the western Soutpansberg but across the mountain range to aid our understanding particularly of the population status of the Near Threatened Cape clawless otter. The Soutpansberg has been identified as an important biological refugium (Hahn, 2011) and could play a key role in protecting Cape clawless otter populations in far northern South Africa.
Acknowledgements: Céline Haring acknowledges Gilian van Duijvendijk from HAS University of Applied Sciences in the Netherlands for internship guidance and advice. Additionally, we thank Jan Crafford for allowing access to his property for conducting this study and Jabu Linden for input on earlier versions of the manuscript. Birthe Linden acknowledges funding from the South African National Research Foundation (grant no. 87311). The support of the National Research Foundation (NRF) through the South African Research Chairs Initiative (SARChI) Chair on Biodiversity Value & Change, hosted at University of Venda, is acknowledged by SW. Lastly, we thank Adrien Alejandro, a research assistant on the project, for running camera traps in the reserve to capture video records of Cape clawless otters resulting in the supplementary video of a mother and her pup.
REFERENCES
Allan, J.D. and Flecker, A.S. (1993). Biodiversity conservation in running waters. BioScience 43: 32-43
Baker, C.M. (1989). Feeding habits of the water mongoose (Atilax paludinosus). Z. Saugetierkd 54: 31-39
Baker., C., Stuart, C., Stuart, M., Nquinana, A., Peinke, D., Maddock, A.H., Perrin, M.R., Somers, M.J., Do Linh San, E. (2016). A conservation assessment of Atilax paludinosus. In: Child, M.F., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H.T. (Eds). The Red List of Mammals of South Africa, Swaziland and Lesotho. South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa
Balian E.V., Lévêque, C., Segers, H. and Martens, K., (Eds.) (2008). Freshwater Animal Diversity Assessment. Hydrobiologia 595
Butler, J.R.A. (1994). Cape clawless otter conservation and a trout river in Zimbabwe: a case study. Oryx 28: 276-282
Butler, J.R.A. and Du Toit, J.T. (1994). Diet and conservation status of Cape clawless otter in eastern Zimbabwe. S. Afr. J. Wildl. Res 24: 41-47
Curagati, C. (1995). Habitat, prey and area requirements of Aonyx capensis and Lutra maculicolis in the Natal Drakensberg. M.Sc Thesis, University of Natal, South Africa.
Do Linh San, E., Angelici, F.M., Maddock, A.H., Baker, C.M. and Ray, J. (2015). Atilax paludinosus. The IUCN Red List of Threatened Species 2015: e.T41590A45204865. https://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T41590A45204865.en (Accessed on 18th August 2022)
Donnelly, B.G. and Grobler, J.H. (1976). Notes on food and anvil using behaviour by the Cape clawless otter, Aonyx capensis, in the Rodes Matopos National Park, Rhodesia. Arnoldia 7 (37): 1-8
Dudgeon, D., Arthington, A.H., Gessner, M.O., Kawabata, Z.I., Knowler, D.J., Lévêque, C., Naiman, R.J., Prieur-Richard, A.H., Soto, D., Stiassny, M.L.J. and Sullivan, C.A. (2005). Freshwater biodiversity: importance, threats, status and conservation challenges. Biology Reviews Biol. Rev. 81: 163-182
GBIF.org (2022a). The Global Biodiversity Information Facility, GBIF Occurrence. https://doi.org/10.15468/dl.9nda55 (Accessed 1st August 2022).
GBIF.org (2022b). The Global Biodiversity Information Facility, GBIF Occurrence. https://doi.org/10.15468/dl.rmx8y9 (Accessed 1st August 2022).
Green, P.A., Vorosmarty, C.J., Harrison, I., Farrell, T., Saenz, L., and Fekete B.M. (2015). Freshwater ecosystem services supporting humans: Pivoting from water crisis to water solutions. Global Environ. Chang. 34: 108-118
Guertin, D.A., Harestad A.S. and Elliot J.E. (2010). Summer Feeding of River Otters Inhabitating a Contaminated Coastal Marine Environment. Northwest Sci 84 (1): 1-8; pp. 4
Hahn, N. (2011). Refinement of the Soutpansberg Geomorphic Province, Limpopo, South Africa. T. Roy. Soc. S. Afr. 66 (1): 32-40
Harding, J.S. (2009). Stream Habitat Assessment Protocols for wadeable rivers and streams in New Zealand. University of Canterbury, School of Biological Sciences.
Harrison, P.A., Vandewalle, M., Sykes, M.T., Berry, P.M., Bugter R., de Bello, F., Feld, C.K., Grandin, U., Harrington, R., Haslett, J.R. and Jongman, R.H. (2010). Identifying and prioritising services in european terrestrial and freshwater ecosystems. Biodivers. Conserv. 19(10): 2791-2821
Jacobsen, L. (2004). Otter (Lutra lutra) predation on stocked brown trout (Salmo trutta) in two Danish lowland rivers. Ecol. Freshw. Fish. 14: 59-68; pp. 62-63
Jacques, H., Reed-Smith, J. & Somers, M.J. (2021). Aonyx capensis. The IUCN Red List of Threatened Species 2021: e.T1793A164575819. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T1793A164575819.en . Accessed on 16 March 2023.
Kingdon, J. (1997). The Kingdon field guide to African mammals. Academic Press, San Diego, California.
Kloskowski, J. (2005). Otter (Lutra lutra) damage at farmed fisheries in south-eastern Poland, II: exploitation of common carp Cyprinus carpio. Wildlife Biology Wildlife Biol. 11 (3): 257-261
Kruuk, H. and Goudswaard, P.C. (1990). Effect of changes in fish populations in Lake Victoria on the food of otters (Lutra maculicollis Schinz and Aonyx capensis Lichtenstein). Afr. J. Ecol. 28: 322-329
Kundu, N., Mausumi, P. and Sharmistha, S. (2008). East Kolkata Wetlands: A Resource Recovery System Through Productive Activities. In: Sengupta, M. and Dalwani, R., (Eds). Proceedings of Taal2007: the 12th World Lake Conference, 868-881
Le Maitre, D.C., Seyler, H., Holland, M., Smith-Adao, L., Nel, J.L., Maherry, A. and Witthüser, K. (2018). Identification, Delineation and Importance of the Strategic Water Source Areas of South Africa, Lesotho and Swaziland for Surface Water and Groundwater. Report No. TT 743/1/18, Water Research Commission, Pretoria.
Ligthart, M.F., Nel, J A.J. and Avenant, N.L. (1994). Diet of Cape clawless otters in part of the Breede River system. Afr. J. Wildl. Res. 24: 38-39
Maddock, A.H. and Perrin, M.R. (1993). Spatial and temporal ecology of an assemblage of viverrids in Natal, South Africa. Jpn. J. Zool. 229: 227-287
Malmqvist, B. and Rundle, S. (2002). Threats to the running water ecosystems of the world. Environ. Conserv. 29: 134-153
Nel, J.A.J. and Somers, M.J. (2007). Distribution and habitat choice of Cape clawless otters, Aonyx capensis, in South Africa. Afr. J. Wildl. Res. 37: 61-70
Okes, N., Ponsonby, D.W., Rowe-Rowe, D., Avenant, N.L. and Somers, M.J. (2016). A conservation assessment of Aonyx capensis. In Child MF, Roxburgh L, Do Linh San E, Raimondo D, Davies-Mostert HT, editors. The Red List of Mammals of South Africa, Swaziland and Lesotho. South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa.
Palazón, S., Ruiz–Olmo, J. and Gosálbez, J. (2008). Autumn–winter diet of three carnivores, European mink (Mustela lutreola), Eurasian otter (Lutra lutra) and small–spotted genet (Genetta genetta), in northern Spain. Anim. Biosiv. Conserv. 31.2: 37–43; pp. 39-40
Perrin, M.R. and Carugati, C. (2000). Habitat use by the Cape clawless otter and the spotted-necked otter in the KwaZulu-Natal Drakensberg, South Africa. S. Afr. J. Wildl. Res. 30: 103-113
Ponsonby, D.W., Rowe-Rowe, D., Power, R.J. and Somers, M.J. (2016). A conservation assessment of Hydrictis maculicollis. In: Child M.F., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H.T. (Eds). The Red List of Mammals of South Africa, Swaziland and Lesotho. South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa.
Purves, M.G., Kruuk, H. and Nel, J.A.J. (1994). Crabs potamonautes perlatus in the diet of otter Aonyx capensis and water mongoose Atilax paludinosus in a freshwater habitat in South Africa. Z. Saugetierkd. 59: 332-341
Rahel, F.J. (2002). Homogenization of freshwater faunas. Annu. Rev. Ecolsyst. 33: 291-315
Reed-Smith, J., Jacques, H. & Somers, M.J. (2021). Hydrictis maculicollis. The IUCN Red List of Threatened Species 2021: e.T12420A164578992. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T12420A164578992.en . Accessed on 16 March 2023.
Revenga, C., Campbell, I., Abelli, R., De Villiers, P. and Bryer, M. (2005). Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philos. T. R. Soc B 360: 397-413
Revenga, C. and Kura, Y. (2003). Status and Trends of Biodiversity of Inland Water Ecosystems. Secretariat of the Convention on Biological Diversity, Montreal, Technical Series no. 11.
Rowe-Rowe, D.T. (1977). Food ecology of otters in Natal, South Africa. Oikos 28: 210-219
Rowe-Rowe, D.T. (1978). The small carnivores of Natal. Lammergeyer 25: 1-48
Rowe-Rowe, D.T. (1985). Facts about otters. Wildlife Management-Technical Guides for Farmers, Natal Parks Board, Pietermaritzburg 10: 1-2
Rowe-Rowe, D.T. (1991). Status of otters in Africa. Habitat 6: 15-20
Rowe-Rowe, D.T. (1992). Survey of South African otters in freshwater habitat, using signs. S. Afr. J. Wildl. Res. 22: 49-55
Rowe-Rowe, D.T. and Somers, M.J. (1998). Diet, foraging behaviour and coexistence of African otters and the water mongoose. Sym. Zool. S. 71: 216-227
Sala, O.E., Chapin, F.S., Armesto, J.J., Berlow, R., Bloomfield, J., Dirzo, R., Huber-Sandwald, E., Huenneke, L.F., Jackson, R.B., Kinzig, A., Leemans, R., Lodge, D., Mooney, H.A., Oesterheld, M., Poff, N.L., Sykes, M.T., Walker, B.H., Walker, M. and Wall, D.H. (2000). Global biodiversity scenarios for the year 2100. Science 287: 1770-1774
Skinner, J.D. and Smithers, R.H.N. (1990). The Mammals of the Southern African Subregion. University of Pretoria, Pretoria.
Somers, M.J. and Purves, M.G. (1996). Trophic overlap between three syntopic semi-Aquatic carnivores: Cape Clawless otter, Spotted-Necked otter and Water Mongoose. Afr. J. Ecol. 34: 158-166
Statistics South Africa (2012). Census 2011 Municipal report – Limpopo. Report no.: 03-01-57, Statistics South Africa, Pretoria.
Stuart, C.T. (1981). Notes on the mammalian carnivores of the Cape Province, South Africa. Bontebok 1: 1-58
Stuart, C. & Stuart M. (2013). A Field Guide to the Tracks & Signs of Southern, Central & East African Wildlife. Struik Nature, Cape Town.
Turnbull-Kemp, P.ST.J. (1960). Quantitative estimations of populations of the river crab, (Potamonautes perlatus) (M. Edw.), in Rhodesian trout streams. Nature 185: 481
Van der Zee, D. (1982). Density of the Cape clawless otter Aonyx capensis (Schinz, 1982) in the Tsitsikama Coastal National Park. S. Afr. J. Wildl. Res. 12: 8-13
Verwoerd D.J. (1987). Observation on the food and status of the Cape clawless otter Aonyx capensis at Betty’s Bay, South Africa.. Afr. J. Zool. 22: 33-39
Résumé: Répartition et Préférence d’Habitat des Loutres à Joues Blanches du Cap (Aonyx capensis) et des Mangoustes des Marais (Atilax paludinosus) dans le Soutpansberg, en Afrique du Sud
Plus de 84% des écosystèmes fluviaux d’Afrique du Sud sont menacés et, par conséquent, les espèces d’eau douce telles que la loutre à joues blanches du Cap (Aonyx capensis) et la mangouste des marais (Atilax paludinosus) sont également en déclin. Ces espèces partagent un régime alimentaire et une préférence d’habitat similaires et, dans certaines régions d’Afrique du Sud, elles se coexistent en sympatrie. Notre étude s’est concentrée sur un système fluvial vierge dans l’extrême ouest du Soutpansberg où l’on sait peu de choses sur la distribution locale et les préférences d’habitat de ces espèces. Pour déterminer la distribution et les préférences d’habitat à petite échelle des loutres et des mangoustes des marais, des Traces et des Signes de présence (TS) et des pièges photographiques ont été utilisés. Le contenu des épreintes a été analysé pour établir les différences de régime alimentaire. Sur la base des TS qui ont été trouvés, la loutre à joues blanches du Cap et la mangouste des marais sont toutes deux largement distribuées le long du système fluvial et vivent principalement séparées l’une de l’autre. La quantité observée de TS de loutres à joues blanches du Cap était plus élevée dans les zones avec des mares, des berges rocheuses et des zones avec une largeur de cours d’eau de 2 m à plus 5 m alors que Le nombre de TS de mangoustes des marais enregistré était plus élevé dans les zones humides avec des berges feuillues et des zones avec une largeur de cours d’eau allant jusqu’à 2 m. Nous suggérons donc que les loutres à joues blanches du Cap et les mangoustes des marais pourraient éviter une concurrence directe en se répartissant l’habitat dans l’ouest du Soutpansberg.
Revenez au dessus
Resumen: Distribución y Preferencias de Hábitat de las Nutrias sin Uñas del Cabo (Aonyx capensis) y las Mangostas Acuáticas (Atilax paludinosus) en el Soutpansberg, Sudáfrica
Más del 84% de los ecosistemas fluviales de Sudáfrica están amenazados y, concordantemente, las especies dependientes del agua dulce como la Nutria sin uñas del Cabo (Aonyx capensis) y la mangosta acuática (Atilax paludinosus) también están declinando en abundancia. Estas especies comparten una dieta y preferencias de hábitat similares, y en algunos lugares de Sudáfrica se sabe que ocurren en simpatría. Nuestro estudio se enfocó en un sistema fluvial pristino en el Soutpansberg más occidental, donde se sabe poco acerca de la distribución local y preferencias de hábitat de éstas especies. Para determinar la distribución y preferencias de hábitat a escala fina de las nutrias y las mangostas acuáticas, se usaron huellas y signos y cámaras-trampa, y se analizó el contenido de fecas para establecer diferencias en la dieta. En base a las huellas y signos que encontramos, la nutria sin uñas del Cabo y la mangosta acuática están ampliamente distribuidas a lo largo del sistema fluvial, y ocurren más que nada separadamente una de otra. La cantidad observada de huellas y signos de las nutrias sin uñas del Cabo fue mayor en áreas con piletones, barrancas fluviales rocosas y áreas con un ancho del curso de agua entre 2 y 5 m de diámetro. La cantidad de huellas y signos de mangosta acuática registrados fue mayor en áreas de humedales con barrancas fluviales con vegetación y hojarasca y áreas con un ancho del curso de agua de hasta 2 m. Sugerimos que las nutrias sin uñas del Cabo y las mangostas acuáticas pueden evitar la competencia directa mediante la partición del hábitat en el Soutpansbereg occidental.
Vuelva a la tapa