IUCN/SSC Otter Specialist Group Bulletin

|
©IUCN/SCC Otter Specialist Group
Volume 33 Issue 1 (January 2016) Citation: Roberts, NJ, Clark, RM and Williams, D (2016). Otter (Lontra longicaudis) Spraint and Mucus Depositions: Early Ecological Insights into the Differences in Marking Site Selection and Implications for Monitoring Prey Availability . IUCN Otter Spec. Group Bull. 33 (1): 8 - 17 Otter (Lontra longicaudis) Spraint and Mucus Depositions: Early Ecological Insights into the Differences in Marking Site Selection and Implications for Monitoring Prey Availability Nathan James Roberts1,2, Ryan Matthew Clark3,4 and Delyth Williams2
1Five Sisters Zoo, Gavieside, West Calder, West Lothian, EH55 8PT
Email: research2@frontier.ac.uk Telephone: +44(0) 207 613 2422 |



|
| (16th January 2015, accepted 10th November 2015)) |
| Abstract: Otters are not territorial in the classical sense of marking territory boundaries. Instead they mark key resource areas within the territory with faeces, or spraint, for olfactory communication. There is a high level of site fidelity in otter marking behaviour and the function of scent marks may explain their spatial distribution. Typically marking sites are in proximity to deep water which provides key resource areas for energy-efficient foraging. Occasionally spraint is laden with mucus, which may also be deposited in isolation without any faecal material. Any difference in the microhabitat variables predisposing site selection by otters with mucus present and absent in depositions has not yet been quantitatively investigated. This study serves as a primary exploration of these selective processes. Here we show that habitat selection by the Neotropical otter (Lontra longicaudis) is different when mucus is deposited at a site compared to when it is absent. The cause of mucus deposition has been suggested in other otter species to indicate reduced prey availability or reproductive state. Depositions here were associated with deep water as in other studies, and temporally, the relative abundance of those with mucus present was highest toward the end of the dry season when prey availability is assumed relatively low. Here we infer how the monitoring of mucus prevalence may be used as a valuable efficient indirect index of the status of otters and their prey. For species whose primary threats include reduced prey availability, such as the Neotropical otter, research attention to these aspects of behavioural ecology are particularly significant in applied conservation. Furthermore, research to validate indirect indices of prey availability and species-habitat interactions may extend to benefit recreational interests and broader human-wildlife conflict mitigation strategies. |
| Keywords: Lontra longicaudis, scent marking, Costa Rica, freshwater, habitat selection, ecological indicators |
| Française | Español |
INTRODUCTION
Olfactory communication is the predominant form of broadcasting information amongst carnivores (Gorman, 1990; Clapham et al., 2014), varying in form and function between species (Smith, 2009). Otters fulfill this communication via scent marks, which are usually deposited in small amounts at selected, repeatedly-used sites rather than simple eliminations of waste as faeces, or spraints (Kleiman, 1966; Hutchings and White, 2000). Though very little is known about the exact messages conveyed in scent marks (Kean et al., 2011), their function may influence their spatial distribution (Ben-David et al., 2005).
Prior to deposition, mucus is added to the spraint (Kruuk, 2006). This mucus is known to also occur in isolation without faecal material suggesting a more complex cause and function than simply as a faeces-binding substance (Erlinge et al., 1982; Davies et al., 1988).
The study of sprainting activity has recently been advocated to give rise to better understanding of behavioural ecology and implications for research design (Rheingantz et al., 2011; Santos and Reis 2012). Further, attention to species-habitat interactions has become increasingly popular in recent years, likely reflecting its pivotal role in applied conservation (Boitani and Powell, 2012).
Our aim here is to quantitatively explore the micro-habitat selective processes acting on the deposition of mucus compared to those for typical spraint marking behaviour by the Neotropical otter (Lontra longicaudis). Our secondary aims are to investigate the level of marking site fidelity and the seasonal variation in detection probability of spraint and mucus depositions.
METHODS
This study was conducted within the Osa Wildlife Refuge on the Osa Peninsula, Costa Rica (83°19’–83°21’ W, 8°23’–8°26’ N; Figure 1). The northern half of the refuge is bordered by the Golfo Dulce Forest Reserve, an area of almost 58,000 ha connecting the neighbouring biodiversity-rich Corcovado National Park with mainland counterparts of the Osa Conservation Area (ACOSA). The average temperature is 26 – 28 °C and annual rainfall is 3,500 – 5,000 mm yr-1, heaviest between late August and early November; the dry season extends from late November through April.
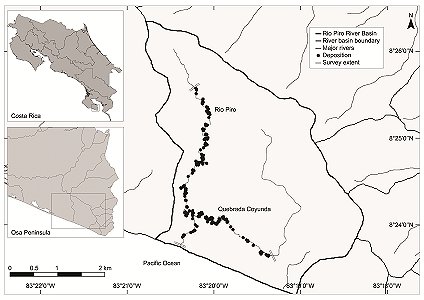 |
| Figure 1. Study site of the Rio Piro River Basin, Osa Peninsula, Costa Rica, presenting all records of depositions including those with mucus present. (click for larger version) |
The intensive study area was within the Rio Piro River Basin (~ 1,500 ha) on the Southern Pacific Coast. The two rivers which form the main drainage of the area, the Rio Piro (5.6 km) and Quebrada Coyunda (6.6 km) reach a confluence less than one kilometre from the ocean, are mostly surrounded by dense forest and have a maximum elevation within the studied area of 160 m above sea level.
We established two linear transects of 5.2 km and 2.8 km on the Rio Piro and Quebrada Coyunda respectively, because of their heterogeneous river profiles within and between the two rivers, and the study area was sufficiently large to encompass temporal changes in distribution. We conducted weekly surveys on foot between November 2013 and June 2014 to encompass periods of variable water levels in response to rainfall; due to these replicates, a total length of 220 km was sampled within the survey period. We surveyed continuously on each sampled morning, scanning for spraints and mucus depositions.
Only spraints less than 48 hrs old were considered, identified by moisture content, colour and stage of decomposition: dry and/or crumbling fading grey-white spraints were disregarded owing to the greater chance of environmental changes occurring between the time of deposition and the time of recording. Secondly, this exclusion of old spraints enabled us to carry out more reliable analyses of temporal variation in sprainting activity, reporting changes in activity rather than frequency of weather-tolerant spraints. Further, we recorded the presence of mucus within spraint or as a separate deposition, and thereby studying only fresh depositions reduced the chance of recording false absences as mucus became dry and undetectable over time.
We recorded each site of deposited spraint and/or mucus as new on its first encounter and as reutilised for every recorded use thereafter. This status was determined by author memory verified with GPS data recorded at every observation and photographs of the sites taken for increased verification power. When a deposition occurred within one metre of another of the same age, we pooled it as one record.
For each record we measured the following variables at the point of deposition, perpendicular to river flow: river width; escape cover distance (ECD) on both sides of the river (distance from the water edge to vegetation line, Anoop and Hussain 2004), and; water depths at 1-m intervals along the river cross section. We determined these variables to be potential factors influencing marking site selection following consultation with published literature and a four-month preliminary study.
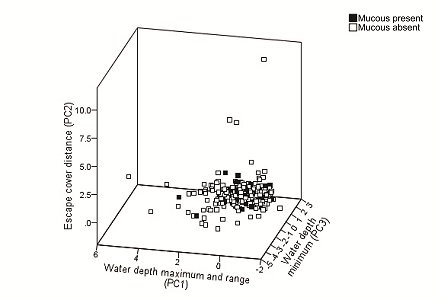
We analysed the spatial distribution of data by average nearest neighbour on ArcGIS 10 using an input area value of 1 km2 to account for river width and a buffer width representing GPS accuracy. Following Anoop and Hussain (2004), principal component analysis (PCA) extracted nine habitat variables from descriptive statistics of ECD and water depth, plus river width (Table 1) which we plotted in a three-dimensional scatterplot for further exploration; we used magnitude and direction (+ or -) of PCA scores to interpret results. We analysed the correlation of variables with Spearman’s rank. We compared measured variables and the Bartlett factor analysis scores computed by PCA with Mann-Whitney U-tests. We performed logistic regression with habitat variables as the covariates and either mucus presence/absence or new/reutilised site status as the dependent variable. We tested data for normality using Shapiro-Wilk test and ANOVA or Kruskal-Wallis tests to infer differences. We performed statistical tests using SPSS 20.
RESULTS
In total, 356 fresh depositions were recorded within the sampled 220 km (Figure 1). Fifty one of these contained mucus and 283 occurred at a reutilised site. Spatial distribution of depositions (mucus and non-mucus pooled) was highly clustered (P<0.01) suggesting the prevalence of micro-habitat selection processes.
Principal component analysis extracted four components with eigen values > 1.0, summarising 89.8% of the variation (Table 1; Figure 2). PC1 (35.6 % of the variance) was related to water depth characteristics favouring sites with deeper water within the cross section: maximum depth and depth range were highly correlated (r = 0.924, P<0.01).
A difference between the selective pressures of mucus present/absent records was statistically validated for PC1 (U=6388, Z=-2.011, P=0.044); other components were not different (U=6436, Z=-1.941, P=0.052 for PC2; U=7651, Z=-0.149, P=0.882 for PC3). Records of mucus deposition carried a higher loading for escape cover distance than river depth for PC1, and vice versa where mucus was absent.
Further statistical analyses revealed that habitat characteristics were not different between sites with mucus present and absent (P>0.2 for all measured variables).
A logistic regression model was developed to predict the presence of mucus and deposition at reutilised sites (Table 2). Of the 51 records found positive with mucus, none were classified with higher probability of presence. Further, none of the mucus-absent records were predicted positive for presence. Only 4.7 % of depositions at new sites were correctly classified; all others were given a higher probability of occurring at reutilised sites.
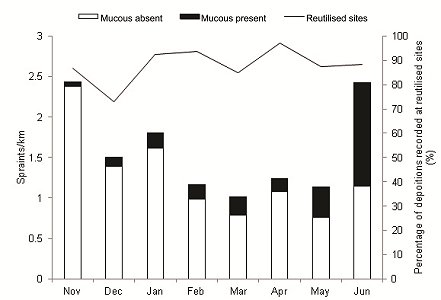
Frequency of spraint depositions per kilometre varied within the study period (F(7, 49) = 3.28, P<0.01) with the greatest frequencies observed in November (2.43 km-1), January (1.81 km-1), and June (2.43 km-1; Figure 3). The relative occurrence of mucus was highest in March (23%), May (33%) and June (53%); mucus deposition per kilometre however, was not statistically different between months (χ2=10.96, df=7, P>0.1). On average, 88 % of depositions occurred at reutilised sites each month.
DISCUSSION
Interpreted as the most important factor influencing marking site selection, water depth likely explains the clustered spatial distribution observed. This association of spraint with deep water concurs with previous studies of L. longicaudis and other otter species (Carrillo-Rubio and Lafón, 2004; Ottino and Giller, 2004; DePue and Ben-David 2010). Extracting whether maximum depth or depth range was more significant was not performed as the two factors were duly correlated owing to cross sections often having at least one depth close to zero centimeters at the measured intervals.
While some aspects of the environment are consistently reported to influence marking site selection, such as water depth, vegetation and conspicuous substrata such as rocky outcrops and fallen trees (Carrillo-Rubio and Lafón, 2004; Kruuk, 2014), it is likely that numerous other interacting factors are at work (Quadros and Montiero-Filho 2002; Depue and Ben-David, 2010). Despite the widespread distribution of the Neotropical otter, the species’ scent marking behaviour has received little research attention (e.g., Carrillo-Rubio and Lafón, 2004; Kasper et al., 2008). A greater understanding of these factors was beyond the scope of this study. Instead the limited number of variables measured here were intended to enable a primary exploration of sites marked with mucus compared with those where mucus was absent, using those factors most consistently reported to be of significant influence.
Indeed this study has revealed different processes acting on the deposition of mucus compared with typical spraint depositions in this case. To our knowledge, this is the first published study to report so distinctly on the occurrence of mucus depositions by Neotropical otters.
For the Eurasian otter (Lutra lutra), these mucus depositions have been associated with food shortages (Kruuk et al., 1993) and for the closely-related North American river otter (Lontra canadensis), it is suggested to signal reproductive state (Rostain et al., 2004). Here, the relative occurrence of mucus was greatest around the period of the end of the dry season when river levels were generally lowest. As fish abundance is expected to be higher in deeper water (Cuplin, 1986), it may follow that the mucus observed here may be a response to reduced prey availability. This interpretation is reinforced by acceptance that food availability likely affects seasonal variation in sprainting behaviour (Melquist and Hornocker, 1983; Ruiz-Olmo et al., 2000; Almeida et al., 2012). Naturally the principal recommendation of this study is to explore relationships between prey availability, river depth and relative occurrence of mucus within a complete annual cycle.
We propose that if mucus occurrence is indeed related to prey availability here as with Eurasian otters (Kruuk et al., 1993), monitoring mucus prevalence may serve as a valuable indirect indicator of the status of otters and their prey. The high level of site fidelity observed here, as reported across other otter species (Erlinge, 1967; Kruuk et al., 1986; Macdonald and Mason, 1986), suggests that such a monitoring program can be highly efficient in terms of deposition detection. Moreover, during periods of heavy rain and when the river mouth became closed to form a lagoon (March – May), some sites became submerged but were marked again once the water level lowered, as also reported by Anoop and Hussain (2004).
Detecting unfavourable changes in prey availability is of particular advantage in the case of the Neotropical otter which is exposed to threats of reduced prey availability (Rheingantz and Trinca 2015). This significance is exacerbated as the dependence on healthy aquatic environments paired with the low reproductive potential of the otter mean the species cannot respond quickly to any ensuing population decline (Cezare et al., 2002; Rheingantz and Trinca, 2015).
Though otters may exploit sub-optimal prey items during periods of reduced prey availability (Kruuk et al., 1993), the reduced abundance of preferred prey is a known causal factor of increased stress and mortality, and reduced immunity and reproductive fitness (Ruiz-Olmo et al., 2001; Nelson et al., 2002; Kitaysky et al., 2010). Further, under conditions of prey stresses, competition between otters and fishing interests can result in harassment and occasional otter mortality (Carrillo-Rubio and Lafón, 2004). Monitoring the status of prey indirectly via mucus, or howsoever else determined, is therefore here attested, yielding both conservation and recreation benefits.
This study represents an early quantitative exploration of the deposition of mucus. It is highly recommended that physiological analyses of mucus are performed to complement field data. Otter spraint is a key resource in itself, increasing heterogeneity and biological productivity at the landscape level such as on the Osa Peninsula (Ben-David et al., 2005; Leuchtenberger et al., 2012; Maslo and Lockwood, 2014). Understanding the cause of mucus depositions and the respective broader implications for conservation and research are encouraged in this and other otter species.
Acknowledgements: This work was carried out on the Frontier Costa Rica Forest Research Programme which is a collaborative initiative between The Society for Environmental Exploration, London and Osa Conservation, Costa Rica. Particular thanks for intellectual and practical input to Sarah Cheetham, Zoe Tarren, Laura King, Emily Williams, Henry Starkie, Noah Ardron, Jennifer Sumners, Beatriz Lopez Gutierrez, Kirsty Hunter, John Scott, Jessamy Bridgewater and all Research Assistants. Thanks also to Jordi Ruiz-Olmo, Rose Argall, Alex Caldwell, Jenna Griffiths and an anonymous reviewer for manuscript suggestions.
REFERENCES
Almeida, D.,
Barrientos, R., Merino-Aguirre, R., Angeler, D. (2012). The role of prey
abundance and flow regulation in the marking behaviour of Eurasian
otters in a Mediterranean catchment. Anim. Behav. 84: 1475–1482.
Anoop, K.R., Hussain, S.A. (2004). Factors affecting
habitat-selection by smooth-coated otters (Lutra perspecillata) in
Kerala, India. J. Zool. 263: 417–423.
Ben-David, M., Blundell, G.M., Kern, J.W., Maier, J.A.K., Brown, E.D.,
Jewett, S.C. (2005). Communication in River Otters: Creation of Variable
Resource Sheds for Terrestrial Communities. Ecology 86: 1331–1345.
Boitani, L., Powell, R.A. (2012). Carnivore Ecology and Conservation: A
Handbook of Techniques. Oxford University Press, Oxford.
Carrillo-Rubio, E., Lafón, A. (2004).
Neotropical river otter
micro-habitat preference in West-central Chihuahua, Mexico.
IUCN Otter
Spec. Group Bull. 21: 10–15.
Cezare, C.H.G., Brandt, A.P., Pianca, C., Josef, C.F. (2002). Some
observations on the southern river otter (Lontra longicaudis, Mammalia:
Mustelidae): status and biology. In: Guix, J.C., Pisciotta, S.K.,
Mateos, E. (Eds.). Censuses of vertebrates in a Brazilian Atlantic
rainforest area: the Paranapiacaba fragment. Centre de Recursos de
Biodiversitat Animal, Universitat de Barcelona, Spain. pp. 149–155.
Clapham, M., Nevin, O.T., Ramsey, A.D., Rosell, F. (2014). Scent-marking
investment and motor patterns are affected by the age and sex of wild
brown bears. Anim. Behav. 94: 107–116.
Cuplin, P. (1986). Aquatic physical features. In: Cooperrider A.Y.,
Boyd, R.J., Stuart, H.R. (Eds.). Inventory and monitoring of wildlife
habitat. U.S. Department of Land Management Service Centre, Denver: pp.
603–612.
Davies, J.M., Lachno, D.R., Roper, T.J. (1988). The anal gland secretion
of the European badger (Meles meles) and its role in social
communication. J. Zool. 216: 455–463.
Depue, J.E., Ben-David, M. (2010). River otter latrine site selection in
arid habitats of western Colorado, USA. J. Wildlife Manage. 74:
1763–1767.
Erlinge, S. (1967). Home range of the otter, Lutra lutra
L., in southern
Sweden. Oikos 18: 186–209.
Erlinge, S., Sandell, M., Brinck, C. (1982). Scent marking and its
territorial significance in stoats, Mustela ermine. Anim. Behav.
30:
811–818.
Gorman, M.L. (1990). Scent-marking strategies in mammals.
Rev. Suisse
Zool. 97: 3–30.
Hutchings, M.R., White, P.C. (2000). Mustelid scent-marking in managed
ecosystems: implications for population management. Mammal Rev.
30:
157–169.
Kasper, C.B., Bastazini, V.A.G., Salvi, J., Grillo, H.C.Z. (2008).
Trophic ecology and the use of shelters and latrines by the Neotropical
otter (Lontra longicaudis) in the Taquari Valley, Southern Brazil.
Iheringia Sér. Zool. 98: 469–474.
Kean, E.F., Muller, C.T., Chadwick, E.A. (2011).
Otter Scent Signals
Age, Sex, and Reproductive Status. Chem. Senses 36: 555.
Kitaysky, A.S., Piatt, J.F., Hatch, S.A., Kitaiskaia, E.V.,
Benowitz-Fredericks, Z.M., Shultz, M.T., Wingfield, J.C. (2010). Food
availability and population processes: severity of nutritional stress
during reproduction predicts survival of long-lived seabirds. Funct.
Ecol. 24: 625–637.
Kleiman, D. (1966). Scent marking in the Canidae.
Sym. Zool. S. 18:
167–177.
Kruuk, H. (2006). Otters; ecology, behaviour and conservation. Oxford
University Press, Oxford.
Kruuk, H. (2014).
Sprainting Into The Wind.
IUCN Otter Spec. Group Bull. 31: 12–14.
Kruuk, H., Conroy, J.W.H., Glimmerveen, E.J., Ouwerkerk, E.J. (1986).
The Use of Spraints to Survey Populations of Otters Lutra lutra.
Biol.
Conserv. 35: 187–194.
Kruuk, H., Carss, D.N., Conroy, J.W.H., Durbin, L. (1993). Otter (Lutra
lutra L.) numbers and fish productivity in rivers in north-east
Scotland. Symp. Zool. Soc. 65: 171–191.
Leuchtenberger, C., Ribas, C., Magnusson, W., Mourão, G. (2012). To each
his own taste: latrines of the giant otter as a food resource for
vertebrates in Southern Pantanal, Brazil. Stud. Neotrop. Fauna E.
47:
81–85.
Macdonald, S.M., Mason, C.F. (1986). Otters, conservation and ecology.
Cambridge University Press, Cambridge.
Maslo, B., Lockwood, J.L. (2014). Coastal Conservation. Cambridge
University Press, Cambridge.
Melquist, W.E., Hornocker, M.G. (1983). Ecology of river otters in west
central Idaho. Wildlife Monogr. 83: 3–60.
Nelson, R.J., Demas, G.E., Klein, S.L., Kriegsfield, L.J. (2002).
Seasonal patterns of stress, immune function, and disease. Cambridge
University Press, Cambridge.
Ottino, P., Giller, P. (2004). Distribution, diet and habitat use of the
otter in relation to land use in the Araglin valley, Southern Ireland.
Biol. Environ. 104B: 1–17.
Quadros, J., Monteiro-Filho, E.L.A. (2002). Sprainting sites of the
neotropical otter, Lontra longicaudis, in an Atlantic forest area of
southern Brazil. J. Neotrop. Mammal. 9: 39–46.
Rheingantz, M.L., Trinca, C.S. (2015).
Lontra longicaudis. The IUCN Red
List of Threatened Species 2015. www.iucnredlist.org. Downloaded on 26
September 2015.
Rheinghantz, M.L., Waldemarin, H.F., Rodrigues, L., Moulton, T.P.
(2011). Seasonal and spatial differences in feeding habits of the
Neotropical otter Lontra longicaudis (Carnivora: Mustelidae) in a
coastal catchment of southeastern Brazil. Zoologia 28: 37–44.
Rostain, R.R., Ben-David, M., Groves, P., Randall, J.A. (2004). Why do
river otters scent-mark? An experimental test of several hypotheses.
Anim. Behav. 68: 703–711.
Ruiz-Olmo, J., Lopez-Martin, J.M., Palazon, S. (2000). The influence of
fish abundance on the otter (Lutra lutra) populations in Iberian
Mediterranean habitats. J. Zool. 254: 325–336.
Ruiz-Olmo, J., Saavedra, D., Jiménez, J. (2001). Testing the surveys and
visual track censuses of Eurasian Otters (Lutra lutra). J. Zool.
253:
359–369.
Santos, L.B., Reis, N.R. (2012). Use of shelters and marking sites by
Lontra longicaudis (Olfers, 1818) in lotic and semilotic environments.
Biota Neotropica 12: 199–205.
Smith, C. (2009). Biology of Sensory Systems. Wiley-Blackwell, New York.
Résumé :
Epreintes de Loutre (Lontra Longicaudis) et Depot de Mucus: Premieres
Considerations Ecologiques des Choix pour la Selection des Sites de Marquage et
les Implications pour Suivre la Disponibilite des Proies
Les loutres ne sont pas territoriales au sens classique du marquage
des limites de leur territoire. Mais elles marquent plutôt les zones de
ressources dans leur domaine vital avec des fèces ou épreintes, pour la
communication olfactive. Le comportement de marquage des loutres montre une
grande fidélité à des sites, et la fonction des marques olfactives peut
expliquer leur distribution spatiale. Les sites de marquage typiques se trouvent
à proximité de zones d’eau profonde qui sont riches en ressources, et rentables
en terme d’énergie dépensée pour les récupérer. Occasionnellement des épreintes
sont recouvertes de mucus qui peut aussi être déposé isolé, sans matériel fécal.
Aucune recherche quantitative dans les différences de l’habitat pouvant
prédisposer les loutres à déposer ou non du mucus n’a été effectuée. Le but de
cette étude est de fournir une première exploration de ce processus de
sélection. Nous montrons ici que la sélection de l’habitat par la loutre à
longue queue est différent quand le mucus est déposé ou non. Des suggestions
pour expliquer le dépôt de mucus chez les autres espèces de loutres comprenaient
une baisse des proies ou un statut reproducteur. Les dépôts ici sont associés
avec une eau profonde comme dans les autres études, et temporairement
l’abondance relative des épreintes avec du mucus était plus importante vers la
fin de la saison sèche quand la disponibilité des proies est moins bonne. Nous
en déduisons que le suivi de la prévalence du mucus peut être utilisé comme un
index indirect mais valable du statut des loutres et de leurs proies. Pour les
espèces dont les principales menaces sont une baisse de la disponibilité de
leurs proies, comme la loutre à loutre queue, les recherches sur ces aspects de
comportement peuvent être importantes dans des stratégies de conservation
appliquée. De plus, la recherche pour valider ces indices indirects de
disponibilité des proies et d’interaction des espèces dans leur habitat peut
être utile à des fins d’activités de loisirs, ou plus largement pour gérer les
conflits faune sauvage/humains.
Revenez au dessus
Resumen: Fecas y Deposiciones Mucosas de la Nutria
Neotropical (Lontra Longicaudis): Primeros Análisis Ecológicos de las
Diferencias en la Selección de Sitios de Marcación e Implicancias para el
Monitoreo de la Disponibilidad de Presas
Las nutrias no son territoriales en el sentido clásico de “marcar los límites
del territorio”. En cambio, marcan áreas de recursos clave dentro de su
territorio, con fecas, para comunicación olfatoria. Hay un alto nivel de
fidelidad de sitio en el comportamiento de marcación de las nutrias, y la
función de las marcas de olor puede explicar su distribución espacial.
Típicamente, los sitios de marcación están en proximidad a aguas profundas que
proveen áreas de recursos clave para una alimentación energeoacute;ticamente eficiente.
Ocasionalmente las fecas están cargadas de mucus, que tambieoacute;n puede ser
depositado solo, sin material fecal. Aún no se ha investigado cuantitativamente
la diferencia en variables de microhabitat que predisponen la selección por
parte de las nutrias, de sitios para dejar deposiciones con o sin mucus. Este
estudio es una primera exploración de estos procesos selectivos. Aquí mostramos
que la selección de hábitat por parte de la nutria Neotropical (Lontra
longicaudis) es diferente cuando se deposita mucus respecto a cuando eoacute;ste está
ausente. Para otras especies de nutria, se ha sugerido que la deposición de
mucus indica reducida disponibilidad de presas, ó estado reproductivo. Aquí, las
deposiciones estuvieron asociadas con aguas profundas como en otros estudios, y
en el aspecto temporal, la abundancia relativa de las deposiciones con mucus fue
mayor hacia el final de la estación seca, cuando se supone que la disponibilidad
de presas es relativamente baja. En este trabajo inferimos cómo el monitoreo de
la prevalencia de mucus puede ser utilizado como un eficiente índice indirecto
del estado de las nutrias y sus presas. Para especies entre cuyas principales
amenazas se incluye la reducción de la disponibilidad de presas, como la nutria
Neotropical, la atención investigativa sobre estos aspectos de la ecología del
comportamiento es particularmente significativa para la conservación aplicada.
Más aún, la investigación para validar índices indirectos de disponibilidad de
presas y de interacciones especie-hábitat, puede extenderse para beneficiar
estrategias de manejo de actividades recreativas ó de mitigación de conflictos
hombre-fauna.
Vuelva a la tapa