IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 35 Issue 1 (January 2018)
Citation: Pinho, FF de, Ferreira, GB and Barata, IM (2018). Feeding Ecology and Spraint Deposition Sites of the Neotropical Otter (Lontra longicaudis) at Cavernas do Peruaçu National Park, Brazil. IUCN Otter Spec. Group Bull. 35 (1): 11 - 21
Feeding Ecology and Spraint Deposition Sites of the Neotropical Otter (Lontra longicaudis) at Cavernas do Peruaçu National Park, Brazil
Fernando Ferreira de Pinho1*, Guilherme Braga Ferreira1,2, 3 and Izabela Menezes Barata1, 4
1 Instituto Biotrópicos, Praça JK 25, Diamantina-MG, Brazil
2Centre for Biodiversity & Environment Research, University College London, Gower Street, London, UK
3Institute of Zoology, Zoological Society of London, Regent`s Park, London, UK
4Durrell Institute of Conservation and Ecology, School of Anthropology and Conservation, Marlowe Building, University of Kent, Canterbury, Kent, UK
* Corresponding Author: fernandopinho@biotropicos.org.br



|
| Download PDF (525 KB) |
| Abstract: Knowledge on the feeding ecology and habitat use of a species is of essential value for effective conservation. We describe the diet and spraints deposition sites for the Neotropical otter (Lontra longicaudis) at Cavernas do Peruaçu National Park, in south eastern Brazil. We collected spraints and recorded characteristics of the deposition sites from 2007–2010. We described otter diet as the number of faeces in which a given taxon was found and the frequency of occurrence of each taxon. We collected 57 spraints and identified 92 food items from nine different taxa, all from animal origin. Fish was the most frequent taxon, found in 98.3% of our samples, followed by arthropods (22.8%) and mammals (10.5%). We recorded 112 spraint deposition sites, most of them located in caves (80%) and <10 m from the water (93.4%). In our study area the Neotropical otter relies heavily on fish, and we believe that the behaviour of some fish species makes them more vulnerable to predation. Habitat use by otters has important management implications for the national park, as caves are the main tourist attraction and some tourist tracks are located next to the river. Although a well-implemented management action might seem enough to avoid negative impacts of tourism, we believe that monitoring the Neotropical otter population in our study area is of major conservation concern to evaluate the impacts of this activity. |
| Keywords: Mustelidae, Lontra longicaudis, feeding ecology, habitat use, faeces, latrine |
| Française | Español | Portugues |
INTRODUCTION
Investigations of Neotropical carnivore feeding ecology are often based on the analyses of faeces (e.g. Garla et al., 2001; Rodrigues, 2002; Novack et al., 2005; Zuercher et al., 2005; Moreno et al., 2006; Massara et al., 2012; Valenzuela et al., 2013; De Angelo et al., 2013; Elbroch and Wittmer, 2013), which can be collected in the species' natural habitat. Faecal collection is considered a non-invasive method that does not harm or stress the animal, and allows the acquisition of valuable data (Perini et al., 2009). This method is ideal for our target species (Lontra longicaudis) because its conspicuous spraints are easily distinguished from other sympatric carnivore species and are usually placed in latrines (Quintela et al., 2011).
The Neotropical otter (Lontra longicaudis) is a carnivorous mammal from the Mustelidae family and is distributed throughout Latin America, from Mexico to Uruguay (Cheida et al., 2011). Neotropical otters are generally diurnal (Parera, 1993) and solitary animals, but can be found in pairs when mating and raising their offspring (Larivière, 1999). The species is well adapted for swimming with a strong tale and fully webbed toes (Cheida et al., 2011), and for these reasons feeds primarily on aquatic animals.
The species is classified as Near Threatened by the IUCN (under criteria A3CD) and its population is thought to be declining (Rheingantz and Trinca, 2015). There is little information available on species' ecology in natural environments and to the best of our knowledge this is the first ecological research on Neotropical otters in the Cerrado-Caatinga ecotone (two major ecosystems in Brazil). In this paper, we describe the diet of Lontra longicaudis and the sites of faeces deposition in Cavernas do Peruaçu National Park, south eastern Brazil.
STUDY AREA
Cavernas do Peruaçu National Park (CPNP), located at Minas Gerais state, south eastern Brazil, encompasses 568 km2 in a transitional area between two major Brazilian ecosystems, the Cerrado and the Caatinga (Fig. 1). Caatinga is a vegetation mosaic constituted of thorn scrub and tropical seasonal dry forests associated with a semi-arid climate, while the Cerrado is a savannah-like ecosystem formed by several vegetation types varying from open grasslands to dense forests.
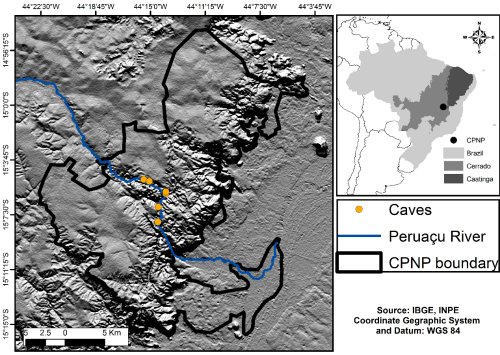 |
| Figure 1. Location of study area at southeastern Brazil, showing the boundaries of Cavernas do Peruaçu National Park and occurrence of caves along the Peruaçu River. (click for larger version) |
The Peruaçu River is the main watercourse in CPNP and runs through a karst area forming a large and deep valley with several caves. In this valley, the river goes across four large caves ranging from 180 m to 4.740 m in extension. Few other small and shallow creeks are present at CPNP, but none of them are likely to support otter populations.
The Peruaçu River valley is mainly represented by riparian and seasonal dry forest - the latter being found further away from the river, especially on the valley slopes. Although selective logging has occurred in some remote areas in the past, most of the valley remained as natural habitats because the topography has discouraged land clearing even prior to the creation of the protected area in 1998.
The annual mean temperature at our study area is 25.2°C and the mean annual rainfall is 805 mm – with some years recording annual rainfall lower than 600 mm, which is classified as semi-arid climate (Geoclock, 2005).
METHODS
Data Collection
We collected Neotropical otter spraints opportunistically between May 2007 and April 2008 while conducting a camera trap survey for large mammals in CPNP. We also did a systematic sampling from 2009 to 2010, when three field trips were conducted at the same areas surveyed in previous years. To collect spraints we walked a continuous track of approximately 10 km along the Peruaçu River searching for potential deposition sites at the river bed and its margin (usually up to 30 m from the river). The area surveyed along the Peruaçu River encompasses most of its extension within the national park (WGS84 -15.1163, -44.2413) and we spent three days in each field trip to cover the entire area. We collected and stored all spraints in individual plastic bags which were labelled with identification and date.
For each spraint deposition site (including those where there were signs of deposition, but no intact spraint) we recorded habitat type (forest or cave) and substrate type (log, sand, rock, or forest floor). Additionally, for spraints found inside caves we also recorded the perpendicular distance from the river. We did not take this measurement in forested habitats because we assumed detection probability would decrease sharply as the distance from the river and vegetation density increased, biasing our data.
We often found the spraints in a naturally dried state, but when needed we used an oven-drying method in the laboratory. We examined all food items under a stereomicroscope and we sorted them into distinct classes: scales, teeth, jaws, hair, bones and carapaces. We used a reference collection and Hildebrand (1995) to identify broad taxonomic groups: Mollusca, Arthropoda, Pisces, Reptilia, Amphibia, Aves and Mammalia. With the help of a specialist and a reference collection from the São Francisco River basin, fishes were further classified into order and, in some cases, identified into genus level.
Data Analysis
Diet composition was described as the number of spraints in which a given taxon was found and also as the frequency of occurrence, dividing the number of total occurrences of each taxon by the total number of spraints collected.
We used Levin's index (Krebs, 1998) as a measure of niche breadth, which ranges from zero to one. According to Krebs (1998), values closer to zero indicate that few items are consumed very often and most items are seldom consumed, while values closer to one represent larger niche breadth where items are more homogenously consumed. The index is given by the formulae:
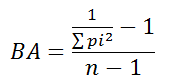
where "BA" is the standardized Levin's index, n is the number of food categories, and pi is the frequency of each food category (Krebs, 1998).
We used a rarefaction curve to evaluate the relationship between the number of spraints collected and the number of taxa identified. This relationship can be interpreted as a measure of sampling completeness of our study, indicating how well our sampling of spraints represents the diet of L. longicaudis at CPNP. For this analysis we used EstimateS 9.1 (Colwell, 2009) software adjusted for 200 randomizations without replacement.
For both the accumulation curve and Levin's index, fishes were classified only to order, because not all samples of this group could be identified to lower taxonomic levels.
RESULTS
Diet of Lontra longicaudis at CPNP
We collected 57 spraints from L. longicaudis and identified 92 food items from nine different taxa, all from animal origin. The average number of taxon identified per spraint was 1.72 (± 0.82). Fish was the taxon most frequently found in the samples, recorded in 56 spraints we analyzed. Arthropods were found in approximately 25% of the samples, while other vertebrates were evident in only 10% or less of the spraints (Table 1).
Fish belonged to three orders, with Characiformes (60%, of which 30/34 were genus Hoplias) and Siluriformes (44%) being most frequently found; (Table 1). Niche breadth as calculated by the Levins' index was 0.1 and the taxa accumulation curve tended to stabilization (Fig. 2).
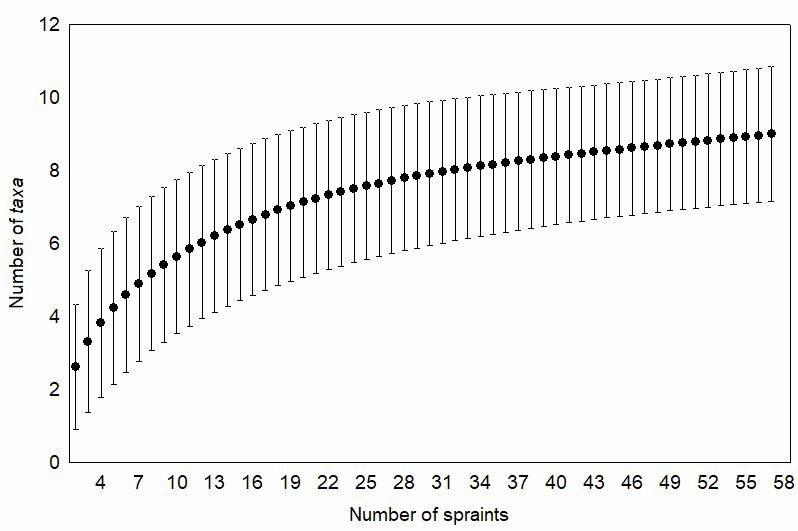 |
| Figure 2. Accumulation curve of taxa identified in relation to the number of Lontra longicaudis spraints collected at Cavernas do Peruaçu National Park, Minas Gerais, south eastern Brazil. |
Spraint Deposition Sites
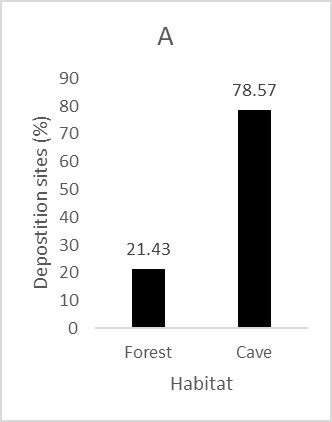
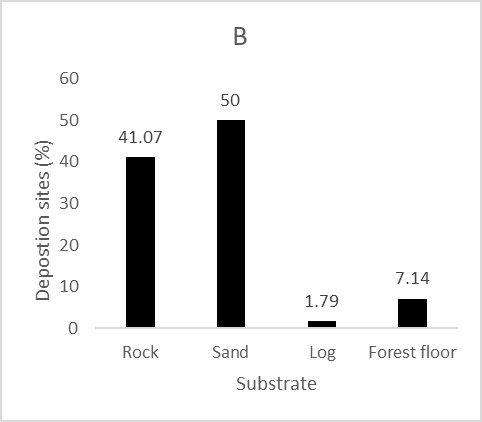
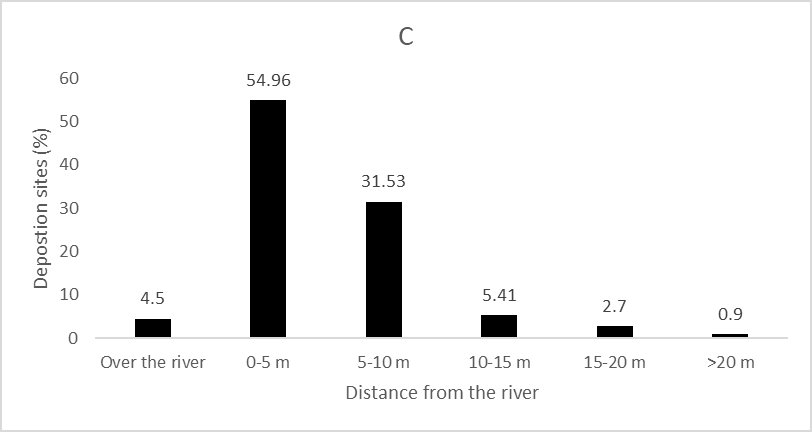
We recorded 112 deposition sites of L. longicaudis next to the Peruaçu River. Caves were largely used by the species as deposition sites with 78.6% of the spraints found in this environment (Fig. 3A). Sand was the substrate most frequently used for deposition, followed by rock and forest floor (Fig. 3B). Most spraints recorded in caves were less than 10 m away from the Peruaçu River (93.4%), with only one sample found further than 50 m from the river (Fig. 3C).
 |
 |
 |
|
| Figure 3. Characteristics of Lontra longicaudis spraint deposition sites by: A) Habitat type; B) Substrate type; C) Distance from Peruaçu River. | |
DISCUSSION
Diet of Lontra longicaudis at CPNP
Our accumulation curve indicates that most taxa consumed by L. longicaudis at CPNP were recorded in our samples, which makes our results representative to characterize the Neotropical otter diet in the study area. The prevalence of fish in the diet of L. longicaudis has been previously observed in several studies in different parts of South America (Pardini, 1998; Colares and Waldemarim, 2000; Brandt, 2004; Kasper et al., 2008; Quintela et al., 2008; Chemes et al., 2010). Only one of our samples did not include fish – as an animal well adapted to aquatic life it is expected that most of the Neotropical otter's prey are aquatic. The same pattern has been observed in the diet of another Neotropical mustelid highly adapted to water, the giant otter (Pteronura brasiliensis) (Rosas et al., 1999; Carter et al., 1999; Cabral et al., 2010).
At CPNP, Characiforme was the most frequent order of fish represented in the diet of L. longicaudis, differing from previous studies (Pardini, 1998; Colares and Waldemarim, 2000; Brandt, 2004; Kasper et al., 2008; Quintela et al., 2008; Chemes et al., 2010). Despite the fact that Brandt (2004) recorded Siluriformes as the most frequent fish item in the diet of L. longicaudis in the Pampa at south Brazil, the frequency of Characiformes was similar to what we observed at the Peruaçu River. Although Neotropical otters can show preferences for some prey items (Pardini, 1998), the composition and structure of the fish community in a given location may have a strong influence on the species' diet. Thus the differences in the frequency of occurrence of fish orders found in our study are probably a consequence of the differences in the fish community in each study area, which are affected by biotic and abiotic features of the particular river basin.
The genus Hopliasis represented in the Peruaçu River by traíras (H. malabaricus) and trairões (H. lacerdae) (Geoclock, 2005), and the high frequency of this food item in the diet of L. longicaudis might be related to the behaviour of these fishes. Hoplias spp. are ambush predators that do not move much in the water (Trajano et al., 2009), and this behaviour might make them vulnerable to be captured by Neotropical otters. Similarly, Pardini (1998) observed that L. longicaudis preferred to feed on prey showing lower escape capability. Moreover, Trajano et al. (2009) reported that Hoplia spp. can live in caves with little light, a habitat type that was frequently used by the Neotropical otter at CPNP.
Fish species from the Characiformes and Siluriformes orders are all native to Peruaçu River, but there is at least one species in the family Cichlidae (order Perciforme) that is exotic to the Peruaçu watershed (Geoclock, 2005). Since the latter family was not identified to genus level we cannot confirm that exotic fish is not part of the Neotropical otter diet at CPNP. However, it is clear that most of L. longicaudis diet in the study area is composed of native fish species.
As observed by Brandt (2004) and Quintela et al. (2008) in the Pampas and by Pardini (1998) in the Atlantic Forest, arthropods were the second most frequent taxon recorded in otter spraints at CPNP. Arthropods, especially crustaceans, are found more frequently than fish in the diet of some otter species (such as the southern river otter Lontra provocax, the marine otter Lontra felina and Asian small-clawed otter Aonyx cinerea) and represent the largest proportion of biomass consumed by these species (Ostfeld et al., 1989; Reyes et al., 2007; Biffi and Iannacone, 2010; Hon et al., 2010).
Although mammals have been recorded in L. longicaudis diet in other studies, it was never reported with a frequency of occurrence greater than 5% (Pardini, 1998; Colares and Waldemarim, 2000; Brandt, 2004; Quintela et al., 2008; Kasper, 2008) - at CPNP mammals represented 10% of items consumed. Despite arthropods occurring more frequently than mammals in the Neotropical otter's diet at our study area, mammals are likely to represent a greater biomass in the species' diet, as all arthropods identified in the spraints had small body size.
Other vertebrate classes (Amphibia, Reptilia and Aves) were recorded in the spraints collected at CPNP, similar to Pardini (1998) and Quintela et al. (2008). Reptiles were not recorded in L. longicaudis diet by Kasper et al. (2008), Brandt (2004) and Colares and Waldemarim (2000), while amphibians were not recorded in the last two studies. None the less these items (especially amphibians) were not frequently observed in the diet of L. longicaudis in our study area. As in the present study, molluscs were recorded in the Neotropical otter's diet at the Pampas (Colares and Waldemarim, 2000; Brandt, 2004; Quintela, 2008) and in the Atlantic Forest (Kasper et al., 2008); however, Mollusca was one of the rarest item consumed, representing a small proportion of food items frequency.
Even though L. longicaudis preyed upon all vertebrate classes and some invertebrates at CPNP, Levin's index indicates a narrow niche breadth, reflecting the high frequency of a few taxa in the diet. Moreover, the presence of the genus Hoplias in half of the spraints collected may even suggest feeding habits of a specialist species. Rheingantz et al. (2012) found that Neotropical otter's diet is more influenced by its specialist feeding behaviour than by the availability of prey. These findings agree with observations that L. longicaudis (Pardini, 1998) as well as other otter species (Van der Zee, 1981; Wise et al., 1981; Kruuk and Moorhouse, 1990) have preference for some prey species or groups.
When investigating otter diet through the collection of spraints, pseudo replication may be an issue because the collection of several samples from few individuals might occur (Quinn and Keough, 2002). We cannot assess the degree of pseudo replication in our data, but we believe that spraints from different individuals were sampled due to the fact that surveys were conducted for a relatively long period (starting in 2007 and finishing in 2010) and approximately 10 km of the Peruaçu River was surveyed. There is no information in the literature about home range size of Lontra longicaudis, but some studies report a density ranging from 0.5 to 2.76 individuals/linear kilometre (Larivière 1990; Kruuk 2006; Carvalho-Junior 1990, 2007 - although these estimates are from other ecosystems). Furthermore, because our survey area encompasses most of the Peruaçu River extension at CPNP, we believe our results are representative of the otter population within the national park.
Spraint Deposition Sites
Some environmental features of the caves at CPNP, especially a sparse (or sometimes, the lack of) vegetation, make it easier to find spraints on these sites, which could potentially lead to a positive bias relative to forest. On the other hand, riparian forests cover a much larger area than caves, and our survey effort (in time and distance walked) was higher in forests when compared to caves. Because we did not control for confounding factors such as detection probability and habitat availability we cannot infer habitat preference. However, the large number of spraints found in caves indicates they are regularly used by Neotropical otters in the study area. The high proportion of spraints found in caves also explains the prevalence of sand and rock substrates for deposition as they are commonly found in this habitat. The presence of Lontra longicaudis in caves was reported in three other regions in Brazil (Dessen et al., 1980; Trajano and Gnaspini-Netto, 1991; Gnaspini and Trajano, 1994), but has not been previously reported for the Peruaçu River. As noted by Pardini and Trajano (1999) in the Betari River at south eastern Brazil, the use of caves by the Neotropical otter in the Peruaçu River is not restricted to the photic zone - spraints were also found in the aphotic zone of caves, where there is no natural light.
Being morphologically adapted to life in the water and feeding mainly on fish it seems natural that most Neotropical otter's spraints were found near the Peruaçu River (<10 m from the water). A similar pattern was recorded for Eurasian otter (Lutra lutra) (Kruuk, 1995) with most spraint deposition sites located next to the river bank. The fact that sites close to the Peruaçu River and caves are frequently used by the species has some management implications for the national park, as these caves are a major tourist attraction in the protected area and some of the tourist tracks are located next to the river. CPNP opened for visitors in 2015 and since then, due to management efforts, a well-organized tourist activity is being conducted in the park: some caves and areas by the Peruaçu River are off-limits for tourists, the number of visitors per day is kept low and the management team is closely monitoring the visits. These actions may be enough to avoid negative impacts from tourist visitation on the Neotropical otter population in the Peruaçu River and should continue in effect. However, it would be important to monitor any negative responses from the species in the following years, since the frequency and number of visitors tends to increase as local tourism sector develops.
Acknowledgements: An award from Conservation Leadership Programme made the 2007-2008 field surveys possible. Leonardo Guimarães Lessa and Maíra Figueiredo Goulart provided valuable comments on an earlier version of this paper and Paulo Pompeu from Universidade Federal de Lavras (UFLA) helped with fish species identification. José Santana kindly guided us at the Peruaçu Valley in our initial survey. ICMBio, the management agency for CPNP, granted us a research permit and provided local support.
REFERENCES
Biffi, D., Iannacone, J. (2010). Variabilidad trófica de Lontra felina (Molina 1782) (Carnivora: Mustelidae) en dos poblaciones de tacna (Perú) entre agosto y diciembre de 2006. Mastozoología Neotropical 17(1): 11-17
Brandt, A. (2004). Dieta e uso do habitat por Lontra longicaudis (Carnivora: Mustelidae) no Parque Estadual de Itapuã, Viamão, RS. Porto Alegre, RS. Dissertação de Mestrado. Universidade Federal do Rio Grande do Sul.
Cabral, M.M., Zuanon, J., Mattos, G.E., Rosas, F.C.W. (2010).Feedinghabits of giant otters Pteronura brasiliensis (Carnivora: Mustelidae) in the Balbina hydroelectric reservoir, Central Brazilian Amazon. Zoologia 27(1): 47-53.
Carter, S.K., Rosas, F.C.W., Cooper, A.B., Cordeiro-Duarte, A.C. (1999). Consumption rate, food preferences and transit time of captive giant otters Pteronura brasiliensis: Implications for the study of wild populations. Aquatic Mammals 25(2): 79-90.
Carvalho-Junior, O. (1990). Aspectos da autoecologia de Lontra longicaudis no ecossistema da Lagoa do Peri, SC, Brasil. Dissertação de mestrado. Universidade Federal de Santa Catarina
Carvalho-Junior, O. (2007). No rastro da lontra brasileira. Ed. Bernuncia, Florianópolis, Brasil. 112p
Cheida, C.C., Nakano-Oliveira, E., Fusco-Costa, R., Rocha-Mendes, F., Quadros, J. (2011). Ordem Carnivora, p.235 – 288. In: Reis, N.R., Peracchi, A.L., Pedro, W.A., Lima, I.P. (Eds.). Mamíferos do Brasil 2ª Ed. Editora da Universidade Estadual de Londrina. Londrina, Brasil.
Chemes, S.B., Giraudo, A.R., Gil, G. (2010). Dieta de Lontra longicaudis (Carnivora, Mustelidae) en el Parque Nacional el Rey (Salta, Argentina) y su comparacion com otras poblaciones de la cuenca del Paraná. Mastozoologia Neotropical 17(1): 19-29.
Colares, E.P.,Waldemarin, H.F. (2000). Feeding of the Neotropical River Otter (Lontra longicaudis) in the Coastal Region of the Rio Grande do Sul State, Southern Brazil. IUCN Otter Specialist Group Bulletin 17 (1): 6-13.
Colwell, R. (2009). EstimateS : Biodiversity Estimation. Diversity 1–23.
De Angelo, C.,Paviolo, A., Rode, D., Cullen, L., Sana, D., Abreu, K.C., Xavier da Silva, M., Bertrand, A.S., Haag, T., Lima, F., Rinaldi, A.R.,Fernàndez, S.,Ramírez, F.,Velàzquez, M., Corio, C., Hasson, E., Di Bitetti, M. S.,Laundré, J.W.,Miotto, R.A.,Cervini, M.,Kajin, M.,Begotti, R.A.,Galetti, P.M., Soto-Shoender, J.R., Main, M.B.,ZanónMartínez, J.I.,Travaini, A., Zapata, S.,Procopio, D., Santillán, M.A. (2013). The ecological role of native and introduced species in the diet of the puma Puma concolor in southern Patagonia. Oryx 47: 109-112.
Dessen, M.B., Eston, V.R., Silva, M.S., Temperini-Becaknd, M.T., Trajano, E. (1980). Levantamento preliminar da fauna de cavernas de algumas regiões do Brasil. Ciência e Cultura 32: 714-725.
Elbroch, L.M., Wittmer, H.U. (2013).The effects of puma prey selection and specialization on less abundant prey in Patagonia. Journal of Mammalogy94: 259-268.
Garla, R.C., Setz, E.Z.F., Gobbi, N. (2001). Jaguar (Panthera onca) food habits in Atlantic Rain Forest of Southeastern Brazil. Biotropica 33(4): 691-696.
Geoclock. (2005). Plano de Manejo do Parque Nacional Cavernas do Peruaçu. Brasília, Brazil.
Gnaspini, P., Trajano, E. (1994). Brazilian cave invertebrates, with a checklist of troglomorphic taxa. Revista Brasileira de Entomologia 38: 549-584.
Hildebrand, M. (1995). Análise da estrutura dos vertebrados. São Paulo. Editora Atheneu. 700 p.
Hon, N.,Neak, P.,Khov, V., Cheat, V. (2010). Food and habitat of Asian small-clawed otters in northeastern Cambodia. IUCN Otter Specialist Group Bulletin 27(1): 12-23.
Kasper, C.B., Feldens, M.J., Salvi, J., Grillo, H.C.Z. (2004). Estudo preliminar sobre a ecologia de Lontra longicaudis (Olfers) (Carnivora, Mustelidae) no Vale do Taquari, Sul do Brasil. Revista Brasileira de Zoologia 21: 65-72.
Krebs, J.K. (1998). Ecological Methodology. 2a ed. Benjamin/Cummings Imprinting. Menlo Park, California, USA. 1998.
Kruuk, H.,Moorhouse, A. (1990). Seasonal and spatial differences in food selection by otters (Lutralutra) in Shetland. Journal of Zoology 221: 621-637.
Kruuk, H. (1995). Wild otters: predation and populations. Oxford University Press. 290p.
Kruuk, H. (2006). Otters. Ecology, behaviour and conservation. Oxford University Press, Oxford, 265p.
Larivière, S. (1999). Lontra longicaudis. Mamm Species 609: 1-5.
Massara, R.,Paschoal, A.M., Hirsch, A.,Chiarello, A.G. (2012). Diet and habitat use by maned wolf outside protected areas in eastern Brazil. Tropical Conservation Science, 5 (3): 284-300.
Moreno, R.S., Kays, R.W., Samudio, R. (2006). Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. Journal of Mammalogy, 87: 808-816.
Novack, A.J., Main, M.B., Sunquist, M.E.,Labisky, R.F. (2005). Foraging ecology of jaguar (Panthera onca) and puma (Puma concolor) in hunted and non-hunted sites within the Maya Biosphere Reserve, Guatemala. Journal of Zoology 267: 1677-178.
Ostfeld, R.S., Ebensperger, L., Klosterman, L., Castilla, J.C. (1989). Foraging, activity budget, and social behavior of the South American Marine Otter Lutra feline (Molina 1782). National Geographic Research 5(4): 442-438.
Pardini, R. (1998). Feeding ecology of the neotropical river otter Lontra longicaudis in an Atlantic Forest stream, south-eastern Brazil. Journal of Zoology 245: 385-391.
Pardini, R.,Trajano, E. (1999). Use of shelters by the neotropical river otter (Lontra longicaudis) in atlantic forest stream, southeastern Brazil. Journal of Mammalogy 80 (2): 600-610.
Parera, A. (1993). The neotropical river otter Lutra longicaudis in Iberá lagoon, Argentina. International Union for the Conservation of Nature. IUCN Otter Specialist Group Bulletin 8: 13-16.
Perini, A.A., Vieira, E.M., Schulz, U.H. (2009.). Evaluation of methods used for diet analysis of the neotropical otter Lontra longicaudis (Carnivora, Mustelidae) based on spraints. Mammalian Biology 74: 290-235.
Quinn, G.P.,Keough, M.J. (2002). Experimental design and data analysis for biologists. Cambridge University Press. 537p
Quintela, F.M., Porciuncula, R.A., Colares, E.P. (2008). Dieta de Lontra longicaudis (Olfers) (Carnívora, Mustelidae) em um arroio costeiro da região sul do Estado do Rio Grande do Sul, Brasil. Neotropical Biology and Conservation 3(3): 119-125.
Reyes, R. (2007). Ecology and Behaviour of the Southern River Otter Lontra provocax (Thomas 1908) in Chile. Dissertação de mestrado. Fachbereich Biologie/Chemie der Universität Osnabrück.
Rheingantz, M.L., Oliveira-Santos, L.G., Waldemarin, H.F., Caramaschi, E. (2012). Are otters generalists or do they prefer larger, slower prey? Feeding flexibility of the Neotropical Otter Lontra longicaudis in the Atlantic Forest. IUCN Otter Specialist Group Bulletin 29(2): 80-94.
Rheingantz, M.L., Trinca, C.S. (2015). Lontra longicaudis. The IUCN Red List of Threatened Species 2015: available in http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12304A21937379.en Downloaded on29 December 2016.
Rodrigues, F.H.G. (2002). Biologia e conservação do lobo-guará na Estação Ecológica de Águas Emendadas, DF. Tese de Doutorado em Ecologia. Universidade Estadual de Campinas.
Rosas, F.C.W., Zuanon, J.A.S., Carter, S.K. (1999). Feeding Ecology of the Giant Otter, Ptenoura brasiliensis. Biotropica 31(3): 502-506.
Trajano, E., Gnaspini-Netto, P. (1991). Fauna cavernícola brasileira, com uma análise preliminar da distribuição dos taxons. Revista Brasileira de Zoologia 7: 383-407.
Trajano, E., Secutti, S., Bichuette, M.E. (2009).Natural history and population data of fishes in caves of the Serra do Ramalho karst area, Middle São Francisco basin, northeastern Brazil. Biota Neotropica 9(1): 128-133.
Valenzuela, A.E.J., Raya, R.A., Fasola, L., Sáenz, R.A.S., Schiavini, A. (2013). Trophic ecology of a top predator colonizing the southern extreme of South America: Feeding habits of invasive American mink (Neovison vison) in Tierra del Fuego. Mammalian Biology, 78: 104-110.
Van Der Zee, D. (1981). Prey of the Cape clawless otter (Aonyx capensis) in the Tsitsikama Coastal National Park, South Africa. Journal of Zoology 194: 467-483.
Wise, M.H., Linn, I.J., Kennedy, C.R. (1981). A comparison of the feeding biology of mink (Mustela vison) and otter (Lutra lutra). Journal of Zoology 195(2): 181-212.
Zuercher, G. L., Gipson, P.S., Carrillo, O. (2005). Diet and habitat associations of bush dogs Speothos venaticus in the Interior Atlantic Forest of eastern Paraguay. Oryx 39(1): 86-89.
Résumé : Ecologie De l'Alimentation et Sites de Marquage d'Épreintes chez la Loutre à Longue Queue (Lontra Longicaudis) dans les Grottes du Parc National dDe Peruaçu au Brésil
La connaissance de l'écologie de l'alimentation et de l'utilisation de l'habitat par une espèce sont essentielles pour une protection efficace. Nous décrivons dans cet article l'alimentation et les sites de marquage d'épreintes chez la loutre à longue queue (Lontra longicaudis) dans les grottes de Peruaçu du parc national au sud est du Brésil. Pour ce faire, nous avons prélevé des épreintes et noté les caractéristiques des sites de marquage entre 2007 et 2010. Nous avons décrit l'alimentation de la loutre ainsi que le nombre d'épreintes dans lesquelles un taxon donné a été trouvé ainsi que la fréquence d'occurence de chaque taxon. Nous avons prélevé 57 épreintes et identifié 92 types d'aliments provenant de 9 taxons différents, tous d'origine animale. Le poisson, taxon le plus fréquent, a été trouvé dans 98,3 % de nos échantillons, suivi par les arthropodes (22,8 %) et les mammifères (10,5 %). Nous avons répertorié 112 sites de marquage d'épreintes, la plupart étant situés dans des grottes (80 %) et à moins de 10 m de l'eau (93,4 %). Dans notre aire d'étude, la loutre à longue queue est fortement dépendante de la présence de poisson, et nous estimons que le comportement de certaines espèces de poissons la rend plus vulnérable à la prédation. L' utilisation des habitats par les loutres a des implications importantes sur la gestion du parc national, notamment les grottes, qui sont l'attraction touristique principale, et la présence de touristes à proximité du cours d'eau. Cependant, un plan de gestion bien adapté devrait être suffisant pour éviter l'impact négatif du tourisme. Nous pensons qu'un suivi de la population de loutre à longue queue dans notre aire d'étude est un élément important de sa protection afin d'évaluer les impacts de cette activité.
Revenez au dessus
Resumen: Ecología Alimentaria y Sitios de Deposición de Fecas de la Nutria Neotropical (Lontra longicaudis) en el Parque Nacional “Cavernas de Peruaçu”, Brasil
El conocimiento de la ecología alimentaria y el uso del hábitat de una especie es de valor esencial para la conservación efectiva. Describimos la dieta y los sitios de deposición de fecas para la nutria neotropical (Lontra longicaudis) en el Parque Nacional Cavernas de Peruaçu, sudeste de Brasil. Colectamos fecas y registramos las características de los sitios de deposición en el período 2007-2010. Describimos la dieta de la nutria como el número de fecas en las que se encontró un taxón dado, y la frecuencia de ocurrencia de cada taxón. Colectamos 57 fecas e identificamos 92 items alimentarios de nueve taxa diferentes, todos de origen animal. El taxón más frecuente fueron los peces, encontrados en 98.3% de nuetras muestras, seguidos por los artrópodos (22.8%) y los mamíferos (10.5%). Registramos 112 sitios de deposición de fecas, la mayoría ubicados en cuevas (80%) y <10 m del agua (93.4%). En nuestra área de estudio la nutria Neotropical se sustenta altamente en base a los peces, y creemos que el comportamiento de algunas especies de peces las hace más vulnerables a la predación. Aunque una acción de manejo bien implementada podría parecer suficiente para evitar los impactos negativos del turismo, creemos que es de importancia primordial de conservación monitorear la población de nutria Neotropical en nuestra área de estudio, para evaluar los impactos de esta actividad.
Vuelva a la tapa
Resumo: Ecologia Alimentar e Sítios De Deposição de Fezes de Lontras Neotropicais (Lontra longicaudis) no Parque Nacioanal Cavernas do Peruaçu, Brasil
Conhecer a ecologia alimentar e o uso do habitat pelas espécies é de essencial importância para ações efetivas de conservação. Neste trabalho descrevemos a dieta e os sítios de deposição de fezes da lontra neotropical (Lontra longicaudis) no Parque Nacional Cavernas do Peruaçu, sudeste do Brasil. Coletamos as fezes e registramos as características dos sítios de deposição entre 2007 e 2010. Descrevemos a dieta como o número de fezes em que cada taxon foi encontrado e a frequência de ocorrência de cada taxon. Coletamos 57 fezes e identificamos 92 itens alimentares de nove taxa diferentes, todos de origem animal. Peixes foi o taxon mais frequente, encontrado em 98.3% das nossas amostras, seguido por artrópodes (22.8%) e mamíferos (10.5%). Registramos 112 sítios de deposição de fezes, a maioria localizados em cavernas (80%) e <10 m de cursos d’água (93.4%). Em nossa área de estudo a lontra neotropical depende muito dos peixes e é provável que o comportamento de algumas espécies de peixes os tornam mais vulneráveis à predação. O uso do habitat pelas lontras tem importantes implicações para o manejo do parque nacional, já que as cavernas são as principais atrações turísticas e algumas trilhas turísticas estão localizadas próximas ao rio. Embora a implantação do turismo no parque nacional esteja sendo feita de forma bem planejada e as medidas adotadas pareçam suficientes para evitar impactos negativos dos visitantes sobre a biodiversidade, acreditamos que o monitoramento da população de lontras neotropicais em nossa área de estudo é de grande interesse para avaliar potenciais impactos futuros dessa atividade
Vuelva a la tapa