IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 36 Issue 1 (January 2019)
Citation: Dognimon, S, Djagoun, C.A.M.S., Djego, S, Akpona, H.A., Djego, J, Akpona, J.D.T. and Sinsin, B (2019).Spotted-Necked Otter (Hydrictis maculicollis) Distribution and Determining Factors of Habitat Occurrence in the Lower Ouémé Valley, Southern Beninn. IUCN Otter Spec. Group Bull. 36 (1): 48 - 60
Spotted-Necked Otter (Hydrictis maculicollis) Distribution and Determining Factors of Habitat Occurrence in the Lower Ouémé Valley, Southern Benin
Samson Dognimon1, Chabi A.M.S. Djagoun1,Sylvie Djego1, Hugues A. Akpona1,2, Julien Djego1, Jean Didier T. Akpona, 3and Brice Sinsin1
1 Laboratory of Applied Ecology, Faculty of Agronomic Sciences, University of Abomey-calavi, 01BP526 LEA-FSA, Cotonou, Benin email: Samsondognimon@gmail.com
2 Direction Générale des Forêts et des Ressources Naturelles, BP. 393 Cotonou, Bénin
3 Université d’Abomey-Calavi Faculté des Sciences Agronomiques, Laboratoire de Biomathématiques et d’Estimations Forestières, 05 BP 1752, Cotonou, Bénin
Received 29th October 2018, accepted 28th January 2019
Abstract: Spotted-necked otters (Hydrictis maculicollis) are present in several major river systems in southern Benin, and their environmental requirements link them to food and water security issues as the region is so densely populated by humans. The lack of baseline data on their distribution and ecology is another major constraint that the species is facing in Benin. The present study aims to determine otter’s distribution and factors affecting the habitat selection in a highly human impacted environment. We conducted a survey on Spotted-necked otter presence/absence in the localities in the lower Ouémé valley in Southern Benin using the non-probabilistic “snowball” sampling method. We then assess the habitat and environmental requirements of Spotted-necked otter from field observations. The spotted-necked otter has shown a wide distribution in southern Benin with the presence signs confirmed in 89% of recorded sites from local perception. According to variables explaining the presence only habitat characteristics such as vegetation cover was significant. The Spotted-necked otter did show a surprising flexibility in their environmental requirements. Our results demonstrate a high adaptability of a threatened carnivore to altered landscapes and show how this flexible behavior opens opportunities for recovery.
Keywords: Spotted-necked otter, Distribution, Habitat choice, Ouémé valley
INTRODUCTION
Increasing population growth and human activities have been shown to highly impact biodiversity worldwide (Vitousek et al., 1997). Due to dietary specialization, their large spatial requirements and low reproduction rate, carnivore species are considered to be especially sensitive to changes in land-use and to human disturbances (Ripple et al., 2014). Consequently, changes in these species behavior are very often adopted as means of adapting to habitat transformation and high levels of human disturbance as a means of surviving. Understanding the adaptability of a species to altered landscapes and its selection of habitats within them are promising to implementation of sustainable conservation measures.
Otters are largely distributed in heavily modified landscapes (Reed-Smith et al., 2015; Ayres and García, 2009). A major cause for the decline in their numbers is attributed to habitat deterioration and loss due to river regulations, dam construction, and modifications to the riparian landscapes (Kruuk, 1995). Additionally, potentially excessive hunting and the growing conflicts due to predation on commercial or subsistence fisheries is taking an unknown toll on all otter species. Changes in habitat structure often alter the availability of resources like food, which in turn requires behavioural plasticity in combination with altered habitat selection or acceptance of novel food resources (Contesse et al., 2004). This raises questions of what kind of habitats they select within anthropogenic altered landscapes.
Some pockets along the Ouémé river in Southern Benin harbour sizeable populations of spotted-necked otter (Hydrictis maculicollis) (S. Djagoun, pers. obs.) and may prove to be a critical area for otter conservation. The Spotted-necked otters is listed on CITES Appendix II (www.cites.org) and classified as a Near-threatened on the IUCN Red List (Reed-Smith et al., 2015) but endangered in Benin Red List (Djagoun et al., 2011). According to Akpona et al., (2011), this species is hunted primarily for food and for products such as skins and organs (for medicinal purposes). Although there is large literature base dealing with the conservation issues (Akpona et al., 2011; Urban et al., 2011; Angelici et al., 2005; Rowe-Rowe, 2016) and conflict aspects (Akpona et al., 2015; Kuhn, 2012; Al-Sheikhly et al., 2014) only a few studies have addressed the ecology of otters in modified landscapes (Bueno-Enciso et al., 2015; Pedroso et al., 2014). There is a lack of understanding on how otters are adapting to the transformation of rivers for human use through changes in riparian vegetation and increased pollution. An anthropogenic environment provides barriers that may limit distribution of the animal. Within the Southern Benin landscape, the lower Ouémé valley exhibits substantial variation in levels of human impact over a small geographic range, best fit to examine otter presence along a gradient of habitat transformation.
This paper aims first to provide a baseline of otter occurrence at the landscape scale and to evaluate the spatio-temporal dynamics of spotted-necked otter according to the local perception and second to characterize the determining factors of spotted-necked otter’s occurrence along river banks the lower Ouémé valley. The hypothesis that modification of aquatic ecosystems severely impacts the distribution and the presence of otters was tested, with the prediction that transformation in the water quality, suitable riparian vegetation, human population density, proximity of the villages and fishing activities would have an adverse impact on otter survival and persistence. We are expecting through this study to generate some data to derive a long‐term monitoring plan for the future conservation of the spotted-necked otter population in Benin.
METHODS
Study Area
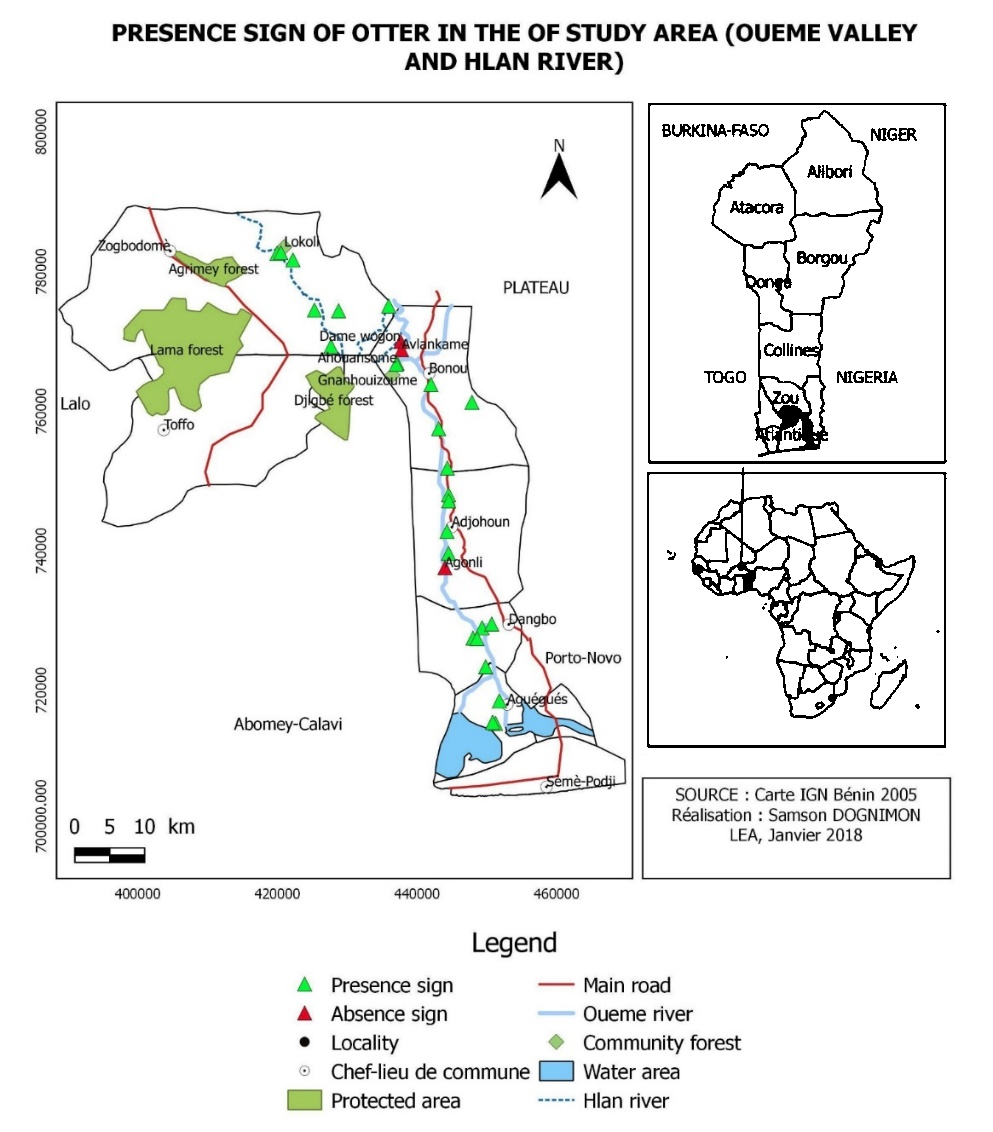
The lower Ouémé valley is located in southern Benin between 6° 24' to 6° 52' latitudes north, and 2° 24' to 2° 38 ' longitude east (Attingli et al., 2016) (Figure 3). The climate is sub-equatorial; its hydrological regime is characterized by two rainy seasons and two dry seasons. Thus, there is a period of low water that usually covesr less seven months (November to June) and a flood period from July to October (Lalèyè et al., 2007). The temperature ranges from 25 °C to 30 °C and the annual rainfall ranges between 900 mm and 1500 mm (Ali et al., 2014). This valley covers an area from Donoukpa in Aguégués municipality to Dame-wogon in Bonou municipality.
The Ouémé valley has large flood plains that ecologically favors many fish species leading to its characterization as a fishery zone (Attingli et al., 2016). The vegetation in the Ouémé valley is composed of herbaceous plants of low grassland with Paspalum vaginatum, Thypha australis and Cyperus papyrus. The vegetation is also composed of floating plants including Eichhornia crassipes (water hyacinth), Pistia stratiotes and Lemne paucicostata (water lettuce). The taxonomic groups of fauna encountered in the Ouémé valley are: mammals, birds, reptiles, amphibians and fish. Mammals include the sitatunga (Tragelaphus spekei), spotted-necked otter (H. macullicolis), mongoose (Crossarchus obscurus), the African manatee (Trichechus senegalensis) and the red-bellied monkey (Cercopithecus erythrogaster erythrogaster) (Kidjo and Guedou, 2001). Local residents are divided in two main ethnic groups: Fon and Weme. The last census of the population (2013) estimated 455,180 inhabitants (INSAE, 2013). This population is much denser in the municipality of Aguégué, which has 856/km² inhabitants followed by that of Dangbo, which is 284 inhabitants /km² and Adjohoun (244 inhabitants /km²). Bonou (161 inhabitants /km²) is the least populated. Fishing and agriculture are the main activities of local residents.
DATA COLLECTION
Firstly, presence/absence data were collected using a semi-structural questionnaire survey technique with the collaboration of the local fishermen living in the potential sites of spotted-necked otter presence according to the literature (Kidjo, 2000; Akpona et al., 2007; 2011; 2015). Local residents of the lower Ouémé valley were interviewed through the "snowball" approach. This non-probabilistic sampling method involved contacting a key person in the population (village chief or president of the fishermen's or hunters' association). This first respondent shows us other key persons who provide us information on the potential occurrence sites of spotted-necked otter in the village or proximate to the village. A total of 263 respondents were interviewed in 28 localities along the lower Ouémé valley in the municipalities of Bonou, Adjohoun, Dangbo and Aguégué as well as some villages along Hlan River in the municipalities of Toffo and Zogbodomè. The interviewees were men, mainly fishermen between 26 and 62 years old.
Secondly, additional data were collected about spatio-temporal dynamics of spotted-necked otter according to local perceptions. Five variables were collected: grouping size, period of observation of otter during the day: night (after 8pm); twilight (6-8 pm); morning (after 7am) dawn (5-6 am) and all time), period of abundance in the year: small dry season (August-September); great dry season (December-March); great rain season (September-November); small rain season (April-July); permanent abundance over the year and the trend of their population over the five last years.

Thirdly, Field observations were made from July to September 2017 in areas listed as having otters present as reported by local perception to confirm the presence of the animal. Indirect observations based mainly on hoop nets attacked (artisanal fishing equipment, Figure 1), footprints (Figure 2) were recorded. According to Akpona et al., (2015), spotted-necked otter actively destroyed the fishing equipment in the lower Ouémé valley. Absence of otter damage in the study localities, combined with the absence of footprints was used to confirm the true absence of the spotted-necked otter in some investigated localities. Additionally we collected some habitat parameters such as: vegetation cover, average height of the vegetation, water depth, water pH, water temperature, population density, number of hoop nets laid per day and nearest distance from village to river bank (Perrin and Carranza, 2000; Anoop and Hussain, 2004) in all investigated sites to generate the drivers of distribution at a local scale within the lower Ouémé valley. The water pH and temperature were measured using HANNA Multimeter and we generated the nearest distance from village to river bank using the QGIS tool. Data on the number of hoop nets laid per day were generated from questionnaires and we used national human census report (INSAE, 2013) to generate the data on the human population density. The vegetation cover and height average were collected within a plot setup along the river bank.
DATA ANALYSIS
Spotted-necked otter’s presence data (GPS coordinates) collected during field surveys were recorded in the QGIS software. These data were projected on the study area map to establish the distribution map of the species.
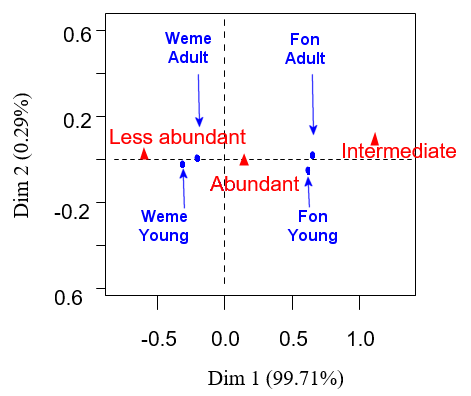
A categorization of respondents according to age was made (Young: <40 years old; Adult: 40 years old and more). Correspondence Analysis (CA) was finally carried out with 'FactoMineR' package (Husson et al., 2014 ) in order to describe graphically the relationships between ethnic group, age group and perceptions on spatial dynamic of the spotted-necked otter. This process helped to know the dynamic of otter population according to ethnic group and age.
The response data collected to determine the ecological and environmental factors affecting the presence of spotted-necked otter is binary. We tested the correlation between the variables with the test of Pearson. A Generalized Linear Model (GLM) based on the binomial distribution was adjusted by using Chi2 adjustment test to the presence data of the otter with the logistic ‘link’ in order to test the influence of eight variables in otter’s presence: vegetation cover, average height of vegetation, water depth, water pH, water temperature, population density, number of hoop net laid per day and nearest distance from village to river bank. The adjusted probabilities were calculated with the ‘epicalc’ package of R software (Chongsuvivatwong, 2012, RCoreTeam, 2017). In this model the response variable is the presence/absence of otter and the independent variables constitute habitat parameters. The result of Pearson’s correlation shows a high correlation between water depth and vegetation (r=-64.79%), also between population density and hoop net laid per day (r=0.60). According to this result and the importance of variables in the explanation of habitat characteristics, we decided that vegetation cover was greater significance than water depth and population density than hoop net laid per day in the analysis. So for the analysis, we take account vegetation cover, height of vegetation, water pH, and water temperature and population density. The model was adjusted to the significant variables (P<0.05).
RESULTS
Distribution of Spotted-Necked Otter in Southern Benin
The spotted-necked otter has a wide distribution in the study area (Figure 3) . A total of 89% of the surveyed areas show the presence signs of the species and hoop net damage was recorded in all sites (Figure 1).
Local Perceptions of Spatio-Temporal Dynamics of Spotted-Necked Otters
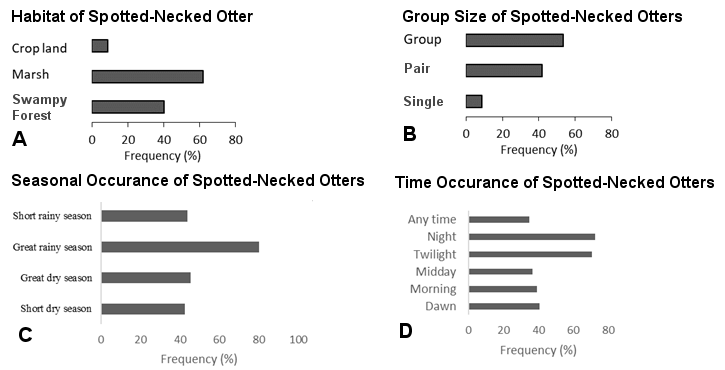
The perceptions of the habitat, seasons, group composition, and periods of occurrence of the spotted-necked otter are presented in Figure 4.
Spotted-necked otter was reported in marsh (62% of the respondents), in swampy forest (40%) and on crop land (9%). According to respondents, the animal is abundant especially during the great rainy season (80%), often in group (54%) or in pairs (42%) and rarely solitary (9%). The most reported occurrence periods were twilight (71%) and night (73%).
Abundance of Spotted-Necked Otter according to Local Population
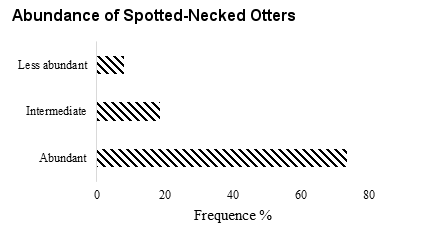
According to most respondents (Ouémé valley and Hlan river), is more abundant compared to the five previous years (Figure 5). As for trend, most respondents said that spotted-necked otter population is abundant (74%) and others respondents (18%) thinks that it is the same in recent years (Figure 5). The results of the CA performed on respondents’ perceptions of the spatio-temporal dynamics show that 99% of the distribution of perceptions within socio-cultural groups is summarized on the one axis. Projections of social groups in the main axis (Figure 6) show that the low abundance of spotted-necked otter is a perception mainly reported by Weme ethnic group while the abundance is reported by Fon ethnic group.
Factors Affecting the Presence of Spotted-Necked Otters
The result (Table 1) of GLM performed on data collected to determine the ecological and environmental factors affecting the presence of spotted-necked otter results showed that only vegetation cover (P=0.036) influences significantly the presence of otter. The factors like average height of the vegetation (P=0.424), water pH (P=0.855), and water temperature (P=0.519) are not significant.
DISCUSSION
Our results showed otters’ presence signs in most of the sites surveyed. The human populations of these localities have generally confirmed the presence of the species. According to local perceptions, spotted-necked otter is mostly observed in marsh and swampy forest but rarely in crop land. Correspondence analysis showed that the perception of the population trend varies according to the ethnic group. Weme ethnic group thinks that otter population is less abundant, however, the Fon ethnic group believe that this population is abundant. Habitat variables were subjected to logistic regression models to examine the factors associated with the occurrence of the spotted-necked otter. The logistic binary regression shown that only the vegetation cover influences significantly the presence of spotted-necked otter in the study area.
Several factors play a role in the habitat choice, and knowledge of these factors is crucial in the understanding of the behaviour and ecology of otters (Shenoy, 2006). It is known that food availability, good vegetation cover are the most important factor determining otter presence in a given habitat (Nel and Sommers, 2007). In the absence of good cover also can greatly influence the presence or absence of otter. The large distribution of spotted-necked otter in the study area can be explained by the abundance of fish, its principal food (Akpona, 2004) in Ouémé valley. Indeed, the study area is described as a fishing zone because of its ecological characteristics that allow the extensive colonization by fish (Attingli et al., 2016; Perrin and Carranza, 2000). Otters cannot live in the area without suitable prey resources (Ayres and García, 2009). Spotted-necked otter have been seen in marshes of less than three meters (Kintocome, Kodonou and Kessounou) and in swampy forests with trees exceeding eight meters (Gnanhouizoumè, Démin and Hon). Use of similar habitats was reported by Perrin and Carrugati (2000) by spotted-necked otter and African clawless otter in Kwazulu-natal Drakensberg (South Africa). A recent, similar study on other otter species confirmed the proximity of otters’ habitat with water (Romero Suances, 2018). The otters’ presence in marsh and in swampy forests indicates it uses all habitats in the study area. Our study confirmed that otter can use all habitat where the water is fresh and rich in fish.
Sites in which the evidence of presence of the species was not observed are sites where human habitations were located along the river bank (Agonli in Adjohoun municipality) and the vegetation cover was low. Trivedi and Joshi (2018) have observed that otters are not habituated to human presence. Human presence negatively influences spotted-necked otter. However, many otter species are tolerant of some degree of human disturbance, and a few species have adapted to human‐modified systems (Okes and O’Riain, 2017). Human presence alone cannot explain the absence of spotted-necked otters in the area. These otters require good vegetation cover or rocky shorelines to hide in; the absence of good cover also can greatly influence the presence or absence of otters. We also observe in these areas that the depth of the river is great. Nel and Sommers (2007) reported that presence of Aonyx capensis, a species having similar habitat requirements to H. macullicolis, is significantly affected by the depth of river. However, in Lake Victoria the presence or absence of otters did not correlate with water depth but with shoreline cover (Reed-Smith et al., 2015). Most of these sites are in places where the species has been reported as absent by Akpona, (2004). This study was implemented in the rainy season, and due to flooding in the study area, it was very difficult to maximize the observation of the otter sign (such as footprints, anal secretions, and spraint). We suggest more investigations during the dry season to confirm the presence/ absence of otter in the study area.
The frequency of observation in marsh and swampy forest is due to the ecology of a species that needs water, fisheries resources and vegetation cover. Night and twilight have been reported as the times of day when otter is more active. Indeed, these periods correspond with when human activities are less intense. The fishermen would have finished visiting the hoop nets and the animal feels safe to move and look for food without risk of being seen. These results are corroborated by those obtained by Akpona (2004) who noticed that H. macullicolis is active at times when their habitat is quiet and unexploited. The same observations were made by Triplet (2009) in his book on the management of protected areas. Greater abundance of otters was reported during the long rainy season. This period is suitable for the animal’s reproduction (June-August) (Akpona 2004). The use of camera trap in future studies can help to get more information on otter’s activity cycle.
The impressions of the Weme ethnic group (a less abundant otter population) can be explained by the fact that spotted-necked otter is much more dispersed in their part of Ouémé valley. This dispersion is justified by the high fishing intensity by the local population which extendsd to all portions of otter’s habitat (Akpona, 2004). Chettri and Savage (2014) showed, through their study on distribution, a negative correlation between the abundance of otter and intensity of fishing activity. The perception of the abundance of otter in an environment is influenced by habitat characteristics and the frequency of hoop net placement in the area. The thicker of the vegetation and the abundance of fish along the Hlan river would favor the colonization of this area by otters which find the habitat and the necessary food resources for their development and growth.
The correlation between the vegetation cover and the presence of otter revealed by this study is similar to th findings of several other authors (Anoop and Hussain, 2004; Chettri and Savage, 2014). The significance of this variable could be explained by the cryptic behaviour of the species. Otters require dense vegetation cover in order to have a hiding place where they feel safe to rest, groom, and raise their young; their presence in a specific area is often dependent on this (Reed-Smith et al., 2015). Perrin and Carrugati (2000) in Kwazulu-Natal discussed the importance of vegetation cover in the habitat selection by spotted-necked otters, and showed that otters choose habitats with dense vegetation, which can provide them with suitable habitat for feeding, reproduction and the safety necessary to ensure the survival of young otters. Hon et al. (2010) showed that otters preferred to live under the forest canopy more than in the open areas. Aonyx cinereu,s like H. maculicolis, dislike open areas that do not offer any shelter. However, in their study, Ottino and Giller (2004) found no correlation in Lutra lutra between otter signs and vegetation cover.
The influence of “vegetation cover” is clearly demonstrate by otterpresence in the Gnanhouizounmè swampy forest whereas they are absent in surrounding sites. Similarly, footprints have occasionally been observed in other areas with dense vegetation (Sekodji in Adjohoun municipality and Demé in Lokoli Forest). This result has to be consider with caution, as the absence of otter sign does not mean they are not there, only that it is harder to find. Furthermore, the presence of sign in more open areas may only mean otters are passing through, rather than indicating residency. More investigations are needed, using camera traps, to investigate otter habitat occupancy in the study area. A previous study (Urban et al., 2011) has demonstrated that optimal habitats for otter are defined as areas with unregulated rivers with trees and other plants providing good cover. The absence of sign of this small carnivore in the localities of Avlankame and Dame Wogon, where there is low vegetation cover, could confirm the high influence of this factor in the choice of habitat of by the species. The importance of vegetation cover is considered to explain habitat choice and preference in other otter species. Acharya and Lamsal (2010) made similar observations on the occurrence of the Smooth-coated otter (Lutrogale perspicillata) in Nepal. Vegetation cover is one of mains factors affecting habitat selection by smooth-coated otter (L. perspicillata) in India (Anoop and Hussain, 2004). In Pakistan, the study done on the Eurasian otter (Lutra lutra) showed the continued survival of species is due to the protection of vegetation (Ullah et al., 2012). Nawab and Hussain (2012) consur. However, it is important to know that this factor alone cannot explain the choice of otter occurrence sites. In fact, studies made by Perrin and Carrugati (2000) in Kwazulu-Natal have revealed that, apart from the vegetation cover, otters prefer areas with little or no human presence and good quality of the water. Kubheka et al. (2012) remarked that river bank vegetation cover and human disturbance were the main factors determining the presence or absence of H. maculicollis.
The period of this study did not allow us to consider all factors determining habitat of spotted-necked otter like stream substrate, the level of disturbance at each site and the presence of visible pollution. The presence sign used (hoop nets damage) is the limit of this study but the unanimity observed around this criterion as an index of the presence of the otter by the population, makes it possible to consider the results of this study in the development of otter conservation strategies.
CONCLUSION
The success of a species-specific conservation project is related to good understanding of the animals’ distribution, and ecological factors determining its habitat choice. Results of this study showed that spotted-necked otter sign is widely distributed in its geographic range, and locally, its presence is strongly influenced by a high percentage of vegetation cover. For the conservation of this species, it is necessary to protect the vegetation cover by reducing tree cutting and exploitation of grasses in the swampy forests. It would be appropriate for future research to extend the study area to all the wetlands of southern Benin and to conduct a line transect study along the bank to collect data on the factors determining the habitat choice.
REFERENCES
Acharya, P.M., Lamsal, P. (2010). A survey for smooth coated otter Lutrogale perspicillata on the River Barayani, Chitwan National Park, Nepal. Hystrix Italian Journal of Mammalogy. (n.s.).21(2): 203-207.
Akpona, A.H. (2004 ). Facteurs de conservation des loutres au Sud du Bénin: Cas de la forêt classée de la Lama et des corridors avec les zones humides de la vallée de l’Ouémé: Thèse d’Ingenieur agronome, FSA UAC Bénin.pp. 81.
Akpona, H.A., Mensah, G.A., Sinsin, B. (2007). Rôle culturel et importance économique de la loutre à cou tacheté Lutra maculicollis au Sud-Bénin Bulletin de la Recherche Agronomique du Bénin. 57: 52-60.
Akpona, H.A., Sinsin, B. and Mensah, G.A. (2011) Monitoring and Threat Assessment of the Spotted-Necked Otter (Lutra maculicollis) in Southern Benin Wetlands . Proceedings of Xth International Otter Colloquium, IUCN Otter Spec. Group Bull. 28A: 45 - 59
Akpona, A.H., Djagoun, C.A.M.S., Harringtonc, L.A., Kabréd, A.T., Mensah, G.A., Sinsin, B. (2015). Conflict between spotted-necked otters and fishermen in Hlan River,Benin. Journal for Nature Conservation.27: 63–71.
Ali, R., Odjoubere, J., Tente, B.H., Sinsin, B. (2014). Caractérisation floristique et analyse des formes de pression sur les forêts sacrées ou communautaires de la Basse Vallée de l’Ouémé au Sud-Est du Bénin. Afrique SCIENCE. 10(2): 243 - 257.
Al-Sheikhly, O., Haba, M., Barbanera, F. (2014). Otter hunting and trapping, a traditional practice of marsh arabs of Iraq. IUCN Otter Spec Group Bull. 31(2): 80-88
Angelici, F.M., E.Politano, Bogudue, A.J., Luiselli, L. (2005). Distribution and habitat of otters (Aonyx capensis and Lutra maculicollis) in southern Nigeria. Italian Journal of Zoology.72(3): 223-227.
Anoop, K.R., Hussain, S.A. (2004). Factors affecting habitat selection by smooth-coated otters (Lutra perspicillata) in Kerala, India. J. Zool. 263: 417–423.
Attingli, A.H., Zinsou, L.H., Vissin, E.W., Laleye, P.A. (2016). Spatialisation des paramètres physico-chimiques dans les pêcheries de la Basse Vallée de l’Ouémé (sud-Bénin). Journal of Applied Biosciences.105: 10190–10202.
Ayres, C., García, P.(2009). Abandoned clay mines: an opportunity for Eurasian otters in Spain. IUCN Otter Spec. Group Bull. 26(2): 66-71 .
Bueno–Enciso, J., Núñez–Escribano, D., Sanz, J. J. (2015). Cultural transmission and its possible effect on urban acoustic adaptation of the great tit Parus major. Animal Biodiversity and Conservation. 38(2): 221-231.
Carugati, C., Rowe-rowe, D.T., Perrin, M.R. (1995). Habitat use by Aonyx capensis and Lutra maculicollis in the natal drakensberg (south africa): preliminary results. Hystrix. 7 (1-2): 239-242.
Chettri, P., Savage, M. (2014). Distribution survey for otter along a river in central Bhutan. IUCN Otter Spec. Group Bull. 31(2): 65-73
Chongsuvivatwong, V. (2012). epicalc: Epidemiological calculator p. R package
Contesse, P., Hegglin, D., Gloor, S., Botadina, F., Duplazes, P. (2004). The diet of urbant foxes (Vulpes vulpes) and the availability of anthropogenic food in the city of Zurich, Switzerland. Mamm.biol. 69 (2): 81-95
Djagoun, C.A.M.S., Akpona, A.H., Daouda, I. (2011). Petits carnivores:Herpestidae, Mustelidae, Viverridae, Canidae, Felidae et Nandiniidae. In: Protection de la nature en Afrique de l’Ouest: Une liste rouge pour le Bénin (eds. P.Neuenschwander, Sinsin, B and Georgen, G.), pp. 318–330.
Hon, N., Neak, P., Khov, V., Cheat, V. (2010). Food and habitat of asian small-clawed otters in northeastern Cambodia. IUCN Otter Spec. Group Bull, 27(1):12-23
Husson, F., Josse, J, Le, S. and Mazet, J. (2014) FactoMineR: Multivariate Exploratory Data Analysis and Data Mining with R.
http://CRAN.R-project.org/package=FactoMineR
INSAE (2013). Cahier des villages et quartiers de ville, pp. 40.
Kidjo, F.C. (2000). Estimation des indices de présence et étude de la stratégie de protection et de conservation des loutres (Aonyx capensis-Scinz, 1821 et Lutra maculicollis - Lichtenstein, 1835; Lutrinae- Mustelidae) dans les zones humides du Sud- Bénin., 26.
Kidjo, F.C. and Guédou, R. (2001). Inventaire et caractérisation des écosystèmes humides des deux complexes Est et Ouest des zones humides du Sud Bénin. Rapport Groupe Faune reptilienne et mammalienne. PAZH, 14p
Kruuk, H. (1995). Wild otters – predation and populations. Oxford University Press.
Kubheka, S.P., Rowe-Rowe, D.T., Alletson, J.D., Perrin, M.R. (2012). Possible influence of increased riparian activity (stream modification and agricultural intensification) on abundance of South African otters. African Journal of Ecology. 51(2): 288–294.
Kuhn, R. (2012). Loutre et activité aquacole : synthèse des connaissances sur la problématique à l’échelle international (Plan National d’Action en Faveur de la Loutre d’Europe, SFEPM), pp.33.
Lalèyè, P., Ezin, A., Vandewalle, P., Philippart, J.C., Teugels, G.G. (2007). La pêche dans le fleuve Ouémé (Bénin). Journal. Afrotropical. Zoology.,Special issue. pp137-148.
Nawab, A., Hussain, S.A. (2012). Factors affecting the occurrence of smooth-coated otter in aquatic systems of the Upper Gangetic Plains, India. Aquatic Conservation: Marine and Freshwater Ecosystems. 22(5): 616–625.
Nel, J.A.J., Somers, M.J. (2007). Distribution and habitat choice of Cape clawless otters, in South Africa. South African Journal of Wildlife Research. 37(1):61-70.
Okes, N.C., O’Riain, M.J. (2017). Otter occupancy in the Cape Peninsula: Estimating the probability of river habitat use by Cape clawless otters, Aonyx capensis across a gradient of human influence. Aquatic Conservation: Marine and Freshwater Ecosystems. 27(3):706–716.
Ottino, P., Giller, P. (2004). Distribution, density,diet and habitat use of the otter in relation to land use in the Araglin valley southern Ireland. Biology and environment. Proceedings of the Royal Irish Academy, p 104
Pedroso, G.M., van Kessel,C., Six,J., Putnam, D., Linquist, B.A. (2014). Productivity,dynamics and water use efficiency in low- and high-input switchgrass systems. GCB Bioenergy. 6: 704–716.
Perrin, M.R., Carranza, D.I. (2000). Use of space by spotted-necked otters in KwaZulu-Natal Drakensberg, South Africa. S. Afr. J. Wildl. Res. 30(1): 15–21.
Perrin, M.R., Carrugati, C. (2000). Habitat use by the cape clawless otter and spotted-necked otters in KwaZulu-Natal Drakensberg, South Africa. S. Afr. J. Wildl. Res. 30(3): 103-113.
RCoreTeam (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria.
Reed-Smith, J., Jacques, H., Somers, M.J. (2015). Hydrictis maculicollis. The IUCN Red List of Threatened Species.
Ripple, W.J., Estes, J. A., Beschta, R.L., Wilmers, C.C., Ritchie, E. G., Hebblewhite, M., Berger,J., Elmhagen, B., Letnic, M., Nelson, P., Schmitz, O.J., Smith,D.W., Wallach, A.D., Wirsing, A.J. (2014). Status and Ecological Effects of the World’s Largest Carnivores. Science, 343:1241484-11.
Romero Suances, R. (2018). The Recovery of a Coastal Eurasian Otter (Lutra lutra) Population in the Galician Atlantic Islands Maritime-Terrestrial National Park. IUCN Otter Spec. Group Bull. 35 (1): 37– 46.
Rowe-Rowe, D.T. (2016). Densities of Otters in the Drakensberg of Kwazulu-Natal, South Africa. IUCN Otter Spec. Group Bull. 33 (2):64 - 67.
Shenoy, K., Varma, S., Prasad, K. V. (2006). Factors determining habitat choice of the smooth-coated otter, Lutra perspicillata in a South Indian river system. Current science. 91(5):637-643.
Triplet, P. (2009). Manuel de gestion des aires protégées d’Afrique francophone, Paris
Trivedi, K., Joshi, P (2018). Photographic Documentation and Distribution of Smooth-Coated Otter (Lutrogale perspicillata) (Geoffroy 1826) in Surat, Gujarat. IUCN Otter Spec. Group Bull. 35 (1): 31 - 36
Ullah, I., Noureen, U., Arshad, M. and Jadoon, W. (2012). Factors Influencing Distribution of Eurasian Otter (Lutra lutra) in Swat and Dir Districts, Pakistan. IUCN Otter Spec. Group Bull. 29 (1): 24 – 33
Urban, P., Balázs, C., Lantos,I., Gáspár,S., Joó, M., Harmos, K. (2011). Eurasian otter (Lutra lutra) in the central part of the Slovak-Hungarian border area. IUCN Otter Spec. Group Bull, 28(2): 99 – 112
Vitousek, P.M., Harold, A.M., Lubchenco, J., Melillo, J.M. (1997). Human Domination of Earth's Ecosystems. Science, 277 (5325) : 494-499.
Distribution de la Loutre à Cou Tacheté (Hydrictis maculicollis) et Facteurs Déterminants de la Présence d’Habitat dans la Vallée du Bas Ouémé, au Sud du Bénin
Les loutres à cou tacheté (Hydrictis maculicollis) sont présentes dans plusieurs grands réseaux hydrographiques au sud du Bénin. Leurs exigences environnementales les lient aux problèmes de sécurité alimentaire et hydrique, la région étant densément peuplée par l'homme. L'absence de données de base sur leur répartition et leur écologie est une autre contrainte majeure à laquelle l'espèce est confrontée au Bénin. La présente étude vise à déterminer la répartition de la loutre et les facteurs affectant la sélection de l’habitat dans un environnement fortement impacté par l’homme. Nous avons mené une enquête sur la présence / absence de loutres à cou tacheté dans les localités de la vallée du bas Ouémé, au sud du Bénin, à l'aide de la méthode d'échantillonnage non probabiliste "boule de neige". Nous avons ensuite évalué les besoins en matière d’habitat et d’environnement de la loutre à cou tacheté sur base d’observations de terrain. La loutre à cou tacheté a une large distribution dans le sud du Bénin et les indices de présence ont été confirmés dans 89% des sites recensés, suivant une information locale. Selon les variables expliquant sa présence, seules les caractéristiques de l'habitat comme la couverture végétale étaient significatives. La loutre à cou tacheté a fait preuve d'une flexibilité surprenante dans ses exigences environnementales. Nos résultats démontrent une grande capacité d'adaptation de ce carnivore menacé dans des paysages altérés et montrent comment ce comportement flexible ouvre des opportunités de récupération..
Revenez au dessus
Resumen: La Distribución y Determinantes de la Ocurrencia de Hábitat para la Nutria de Cuello Manchado a lo Largo del Valle de Ouémé y el Río Hlan en el Sur de Benin
Las especies de nutria de cuello manchado están presentes en varios sistemas fluviales importantes en el sur de Benin, y sus requisitos ambientales los vinculan con los problemas de seguridad de los alimentos y el agua, ya que la región está tan densamente poblada por humanos. La falta de datos de referencia sobre su distribución y ecología es otra limitación importante que enfrenta la especie en Benin. El presente estudio tiene como objetivo determinar la distribución de la nutria y los factores que afectan la selección del hábitat en un entorno altamente afectado por el ser humano. Realizamos un estudio sobre la presencia / ausencia de nutria de cuello manchado en las localidades del valle inferior de Ouémé, en el Benin, utilizando el método de muestreo no probabilístico de "bola de nieve". Luego accedemos a los requisitos de hábitat y ambientales de la nutria de cuello moteado a partir de observaciones de campo. La nutria de cuello manchado ha mostrado una amplia distribución en el sur de Benin, con signos de presencia que confirman en el 89% del sitio registrado a partir de la percepción local. De acuerdo con las variables que explican la presencia, solo las características del hábitat como la cobertura vegetal fueron significativas. La nutria de cuello manchado mostró una sorprendente flexibilidad en sus requisitos medioambientales. Nuestros resultados demuestran una alta adaptabilidad de un carnívoro amenazado a paisajes alterados y muestran cómo este comportamiento flexible abre oportunidades para la recuperación.
Vuelva a la tapa