IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 1 (January 2021)
Citation: Narasimmarajan, K., Hayward, M.W., and Mathai, M.T. (2021). Assessing the Occurrence and Resource Use Pattern of Smooth-Coated Otters Lutrogale Perspicillata Geoffroy (Carnivora, Mustelidae) in the Moyar River of the Western Ghats Biodiversity Hotspot. IUCN Otter Spec. Group Bull. 38 (1): 43 - 58
Assessing the Occurrence and Resource Use Pattern of Smooth-Coated Otters Lutrogale Perspicillata Geoffroy (Carnivora, Mustelidae) in the Moyar River of the Western Ghats Biodiversity Hotspot
Kannadasan Narasimmarajan1*, Matt W. Hayward2 and Manu Thomas Mathai1
1Department of Zoology, Madras Christian College, East Tambaram, 600059 Chennai, India
2School of Environmental and Life Sciences, University of Newcastle, Callaghan, NSW Australia 2207
* Corresponding Author: e-mail wildlife9protect@gmail.com
(Received 19th February 2019, accepted 9th August 2020)
Abstract: Understanding the factors affecting presence and habitat requirements of threatened species is fundamental to their conservation. Patterns of occurrence and resource use by the vulnerable Smooth-coated otter were examined by sampling Moyar River in the Western Ghats biodiversity hotspot of India between 2015 and 2017 for otter spraints, tracks, dens and grooming sites. An occurrence-based framework was used to determine the influence of environmental covariates on otter detectability. Information on environmental parameters was recorded every 400 meters along the riverbanks in post-monsoon, winter, and summer seasons. Smooth-coated otter occurrence was high before the summer but waned drastically after monsoon when increased water levels reduce detectability of otters by washing away signs of their presence. Otter occurrence and resource use patterns were influenced by river substrate, habitat characteristics, riverbank traits and forest types. Otters prefer wider rivers, but avoided rocky areas with shallow water. Resource use patterns were determined by habitat traits and disturbance in all three seasons. Various forms of disturbances adversely affected the otter occurrence. Restoration of degraded habitats is necessary to improve the long-term conservation prospects of otters. Regional otter conservation plans need to be species-specific to help maintain the ecological balance of Moyar River ecosystem.
Keywords:otter; occurrence; conservation; vulnerable; Moyar river; Western Ghats
INTRODUCTION
Freshwater ecosystems provide a wide range of goods and services, and housing a rich biodiversity (Newman and Griffin, 1994). In many Asian countries, human population levels have steadily increased during the last century; however existing rural development programmes have not adequately addressed key issues, such as poverty, forcing people to become increasingly dependent on rapidly depleting natural resources (Sathyanarayana, 1997). Freshwater ecosystems suffer from increased levels of point and non-point sources of pollution and developments (Raj, 1941). Increased anthropogenic impacts also pose a major threat to species that dependent on freshwater ecosystems (Ruiz-Olmoand Consalbez, 1997).
Otters are often the apex predator in freshwater and wetland ecosystems (Kruuk, 1995 ). However, throughout their ranges, otters are losing their habitat as a result of the construction of dams and barrages, reclamation of wetlands for settlements and agriculture, reduction in prey biomass, poaching, and contamination of waterways by pesticides and weeds (Raj, 1941).These activities have a profound impact on aquatic food webs and the habitat relationships of species occurring there (Hussain et al., 2008).
Smooth-coated otters (Lutrogale perspicillata) are the largest Asian otter species and are categorized as Vulnerable in the IUCN Red List (Nawab and Hussain, 2012a) and are on Appendix II of CITES (Hussain and Choudhury, 1997).The existing populations of the three species of otters in India (the other two being Small-clawed otter (Aonyx cinerea) and Eurasian otter (Lutra lutra)), and their habitat have been surveyed very infrequently (Gupta et al., 2016). The minimal understanding of otter ecology we have is on gross habitat assessments, and there is a suggestion of a rapid decline of otter populations through habitat loss and intensive poaching (Raj, 1941; Hussain, 1996; Hussain and Choudhury, 1997; Anoop and Hussain, 2004; Nawab and Hussain, 2012a,b). Previous work on otters in India has mostly involved observations on captive animals (Hussain and Choudhury, 1997) and occasional notes on their occurrence from different parts of the country (Nawab and Hussain, 2012a).
The Moyar is a significant perennial river in the Western Ghats biodiversity hotspot of India and is thought to support a sizable otter population. However, indiscriminate fishing activities, pumping of river water for irrigation farming, hydro-electric projects and mesquite invasion along the riverbanks are affecting the occurrence and resource use patterns of otters (Narasimmarajan et al., 2018). There is no information on otter occurrence and resource use in this region, which hinders appropriate conservation action being taken.
MATERIALS AND METHODS
Study area
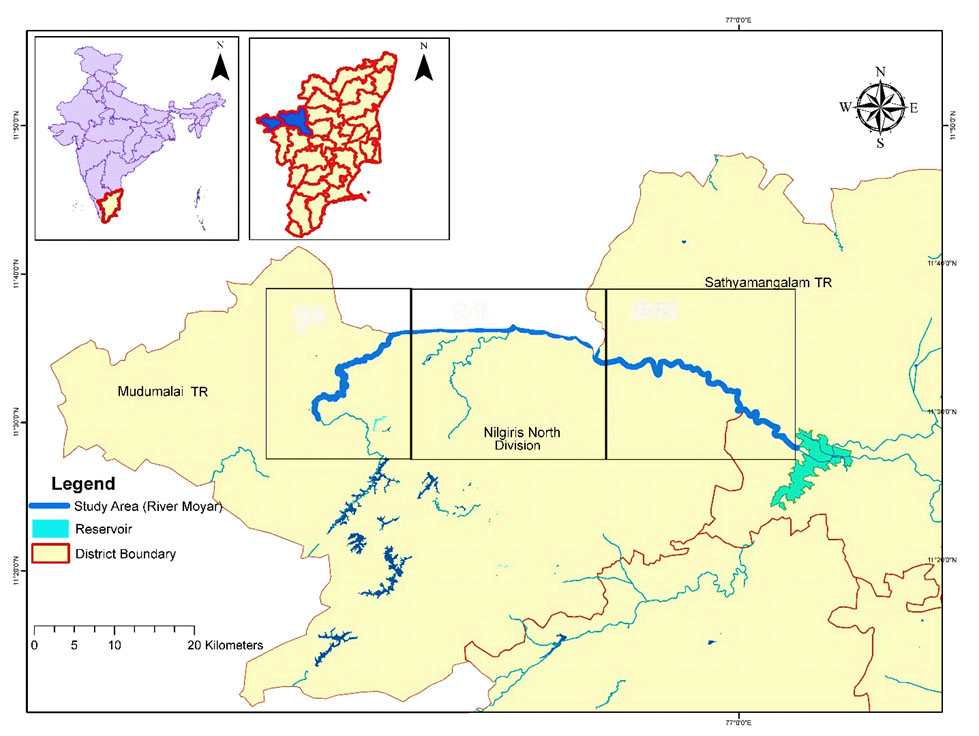
The Moyar River in the Western Ghats biodiversity hotspot in India flows through several protected areas (Mudumalai Tiger Reserve, Sathyamangalam Tiger Reserve, Nilgiri North and South Divisions; Fig. 1).The upper reaches of the river receive ~5,000 mm of rainfall, whereas the down part reaches receive ~824 mm rainfall annually (Puyravaud and Davidar, 2013).The minimum and maximum annual average temperatures in this region vary from 14 ˚C – 30 ˚C in higher elevations, and 25 ˚C – 38 ˚C in the lower elevations (Narasimmarajan et al., 2018). Elevation of the river varies from 250 masl (Bavanisagar Dam) to 2,054 masl (Pykara Dam) (Champion and Seth, 1962). The landscape supports large populations of tiger (Panthera tigris), leopard (Panthera pardus), elephant (Elephas maximus), otters (Lutagale perspicillata; Aonyx cinerea) and endangered vultures (Gyps benghalensis; Gyps indicus; Neophron percnopterus; Aegypius monachus) (Narasimmarajan et al., 2018). The river is an important source of irrigation for thousands of hectares of agricultural land and supports the livelihood of more than a million people (Puyravaud and Davidar, 2013). However, this river ecosystem faces many threats, such as agricultural runoff, hydroelectric projects, unrestricted fishing activities, pesticides and spilling of motor oil (Narasimmarajan et al., 2018). In addition, mesquite continues to invade the river gorges, catastrophically impacting native vegetation (Kumar and Mathur, 2014).
Data collection
We aimed to investigate the distribution and resource use of smooth-coated otters in the river Moyar study region using the survey methods of Hussain and Choudhury (1997); Brzezinski and Jedrzejewska (1993); Anoop and Hussain (2004) and Nawab and Hussain (2012a). The entire River Moyar and its tributaries were divided into 6 km segments (minimum otter home range based on Hussain and Choudhury, 1997) using geographical information systems (Fig. 1). Data on environmental parameters and otter signs (spraints, tracks, dens and grooming sites) were recorded every 400 m (random stratified, at significant crossing/access points) and we consider each 400 m transect a study plot. The data was collected between 2015 and 2017.
A team of four researchers conducted the survey by walking along both riverbanks, searching for otter signs. A few inaccessible areas (n=4) were excluded due to safety concerns. In each survey (post-monsoon, winter and summer) season, the plots where spraints, tracks, grooming plots, dens and other signs of otter presence were found defined as a ‘used/positive sites. A new plot was considered as such only when spraints were separated by >15 m (Melquist and Hornocker, 1983; Newman and Griffin, 1994; Medina, 1996). For the estimation of habitat availability, each plot was categorized as rocky stretch, sandy stretch, muddy stretch, clayey stretch and alluvial stretch.
In each survey season, data on environmental parameters and disturbance that were considered potentially important to otters were collected from each plot (Macdonald and Mason, 1983; Brzezinski and Jedrzejewska, 1993; Anoop and Hussain, 2004). Opportunistic observations of otters during the course of the surveys were also recorded and their group size, structure and activity were noted.
Data analysis
A detection history was created based on whether otter signs were detected (1) or non-detected (0) for each season at each 400 m long study plot. The covariate data collected for available and used plot was organized in sample-habitat parameter matrix for post monsoon, winter and summer seasons respectively. The raw data matrix was arranged into proportionate and continuous data, which had to be suitably transformed via arcsine and log transformation and standardized following Zar (1984). Factor analysis was used to reduce the dimensionality of the habitat variables. The first three factors (predictors) were used for interpretation as these explained maximum variations in the data set, and Pearson product moment coefficient as the input and a varimax rotation of the factors. Simple cross-tabulations and χ2 statistics were calculated for the detection of possible relationships between resource variables (i.e. the presence/absence of different environmental traits, and presence/absence of sprainting activity) and auto-correlated null variables were dropped due to their non-aligned influence on the sprainting activity (Van Emden, 2008).Pearson’s correlation was used to subset the null deviations, and the constant-only model used the significant covariates for further analysis (MacKenzie et al., 2002; Kruuk, 1995). Significant associations between habitat traits, and the presence or absence of spraints are shown in Table 1.
Spraints are likely to be detected more often than expected where rocky and loose sand occurs in the immediate vicinity of a survey plot (White et al., 2003) and may be overlooked in dense vegetation, etc. Spraints are likely to be found less often than expected at plots with grass either in the surrounding land or the immediate vicinity. The land cover variables that were significant were included with those representing stream order, stream gradient and habitat characters in the logistic regression analysis (White et al., 2003).Global logistic regression model (Z-value) including variables relating to habitat, the physical characteristics of the river and surrounding vegetation cover was able to predict the presence or absence of otter sprainting at different survey plots with an accuracy of 92% using software R (R Core Team, 2018).
RESULTS
Resource availability
Fourteen categories of covariates were obtained for the study area. At the landscape level, the mean ± SE of substrate variable ‘hard sand’ constituted 7.40 ± 0.7 followed by rocky stretches composed of boulders 4.51 ± 0.6, loose sand stretches 2.47 ± 0.1, while alluvial stones constituted the least at 1.12 ± 0.3. Habitat variables were consisted of canopy cover 18 ± 2.7%, dry leaf litter cover 6.49 ± 0.9, bank vegetation 4.42 ± 0.4 and average grass height 3.97 ± 0.5. River characteristics represented attitude/elevation 464.5 ± 6.7 followed by water current 11.5 ± 1.9, river depth 2.6 ± 0.1 and river width 2.06 ± 0.3 (Table 2).
Factors influencing the smooth-coated otter occurrence in post-monsoon winter and summer seasons in river Moyar
The principal component analysis had an efficiency of 93.41% of available and utilized plots. The model shows that the smooth-coated otter sprainting activities were positively related to sandy stretches with moderately steep bank slopes, with greater than average numbers of dead logs, and tall average grass height with no disturbance. Otter grooming plots occur along wider rivers at junctions where several streams join the main river, and they are least likely to occur near stagnant water. Conversely, tracks were only found in sandy stretches with no disturbance (Table 3). Sprainting activity varied significantly with seasons and decreased significantly with altitude/elevation and disturbance. The form of these relationships was unimodal.
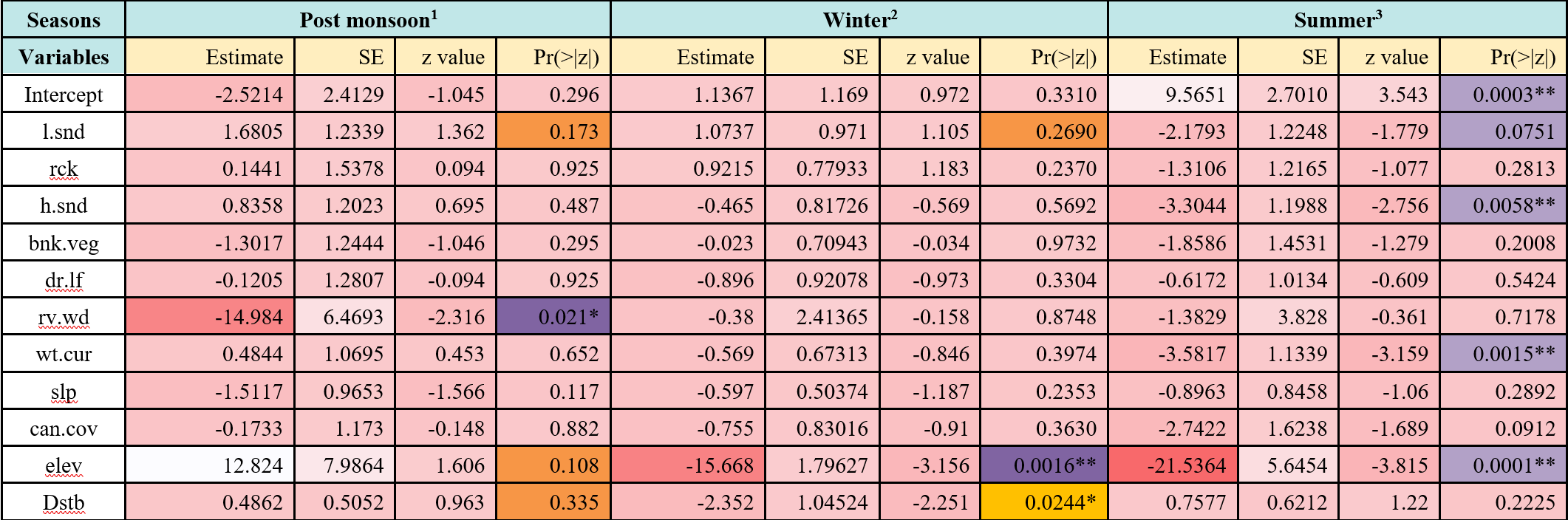
The logistic regression explained 80.23% of the variance in otter presence. The post-monsoon otter occurrence were positively related to rocky stretches (z=0.1441, P<0.01), loose sand (z=1.6805, P<0.01), water current (z=0.4844, P<0.01) and altitude/elevation (z=0.633, P<0.01); but were negatively correlated with river width (z=-14.894, P<0.01) and dry leaf litter cover (z=-0.105, P<0.01). During winter, the otter occurrence were positively related with loose sand (z=1.0737, P<0.01) and rocky stretches (z=0.9215, P<0.01), but were negatively correlated with altitude/elevation (z=-15.668, P<0.01).In summer, otter occurrence was positively related to disturbance (z=0.7577, P<0.01), and negatively related with loose sand (z=-2.1742, P<0.01), water current (z=-3.3044, P<0.01) and altitude/elevation (z=-21.536, P<0.01; Table 4).
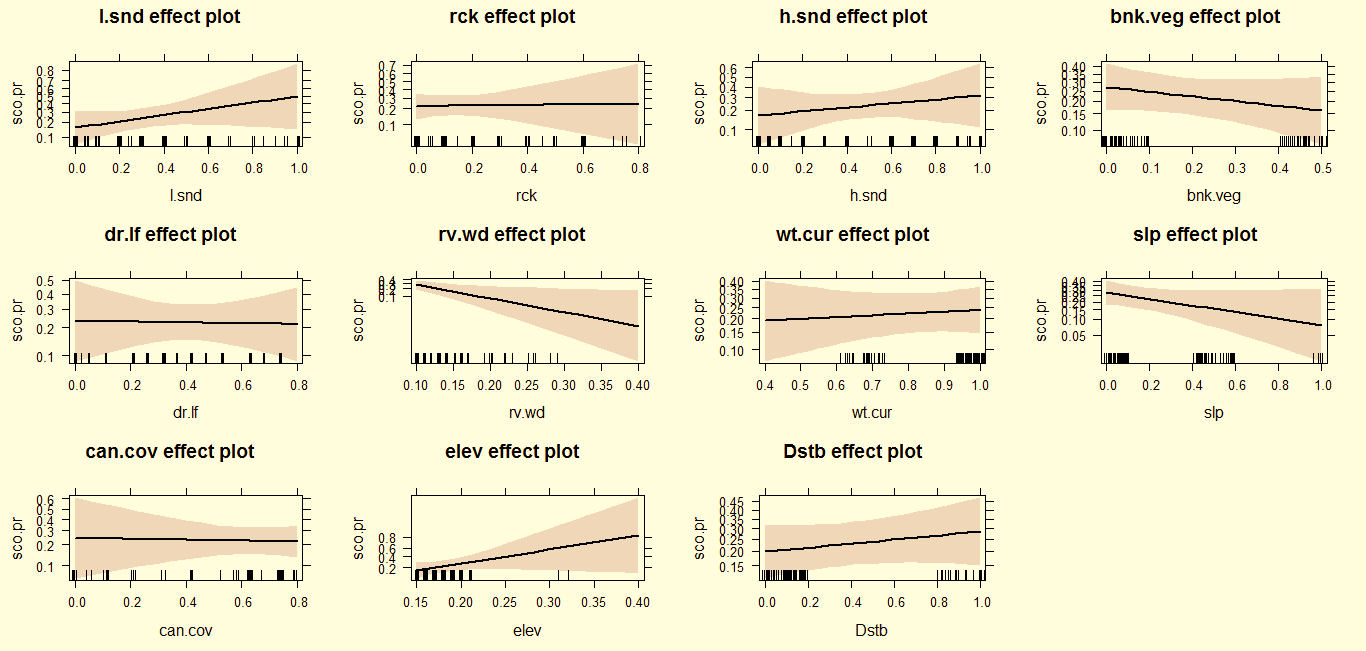
After the monsoon, otters preferred rocky areas with loose sand along with low altitude and fast flowing water current, and avoid narrow river plots, deep slope gorges (>65˚), dense vegetation cover and dry leaf litter cover (Fig. 2). In winter, otters maintain their preferences for rocky areas with loose sand at low altitude, but also avoid disturbance and higher elevation/altitude plots (Fig. 3).In summer, otters coexist with anthropogenic disturbance, but avoid loose and hard sandy areas, fast flowing water, and higher elevations in contrast to previous seasons (Fig. 4).
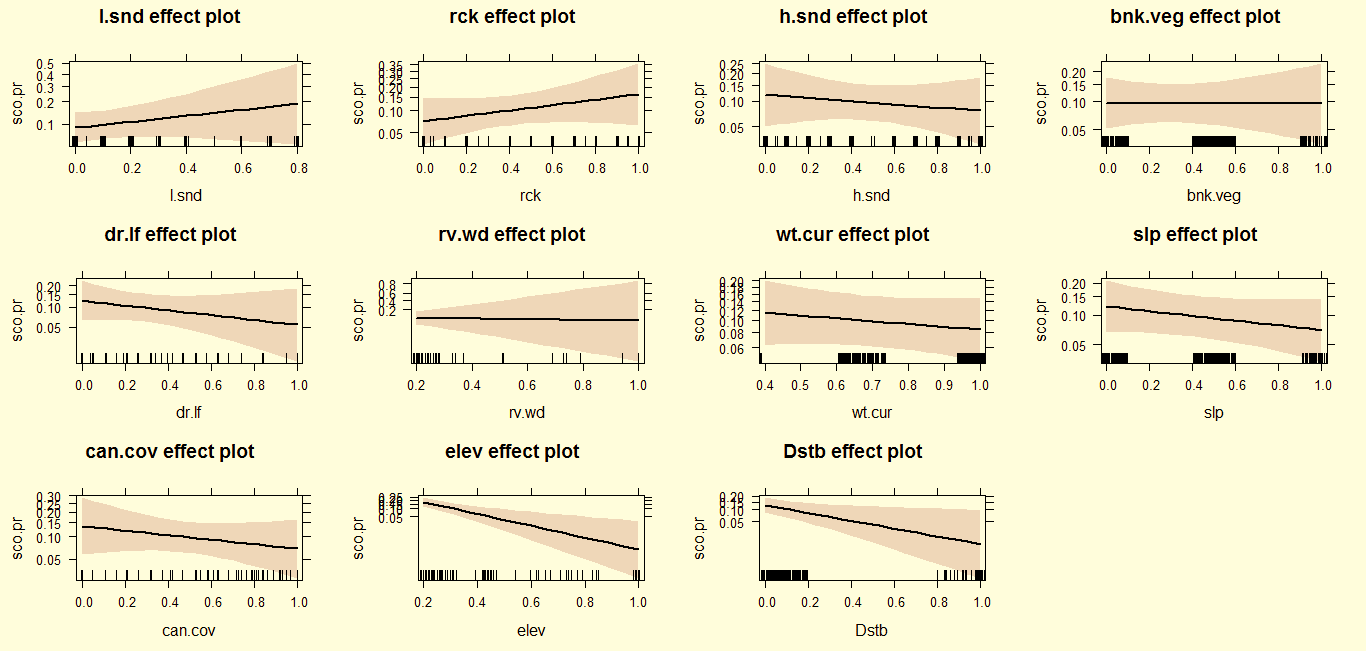
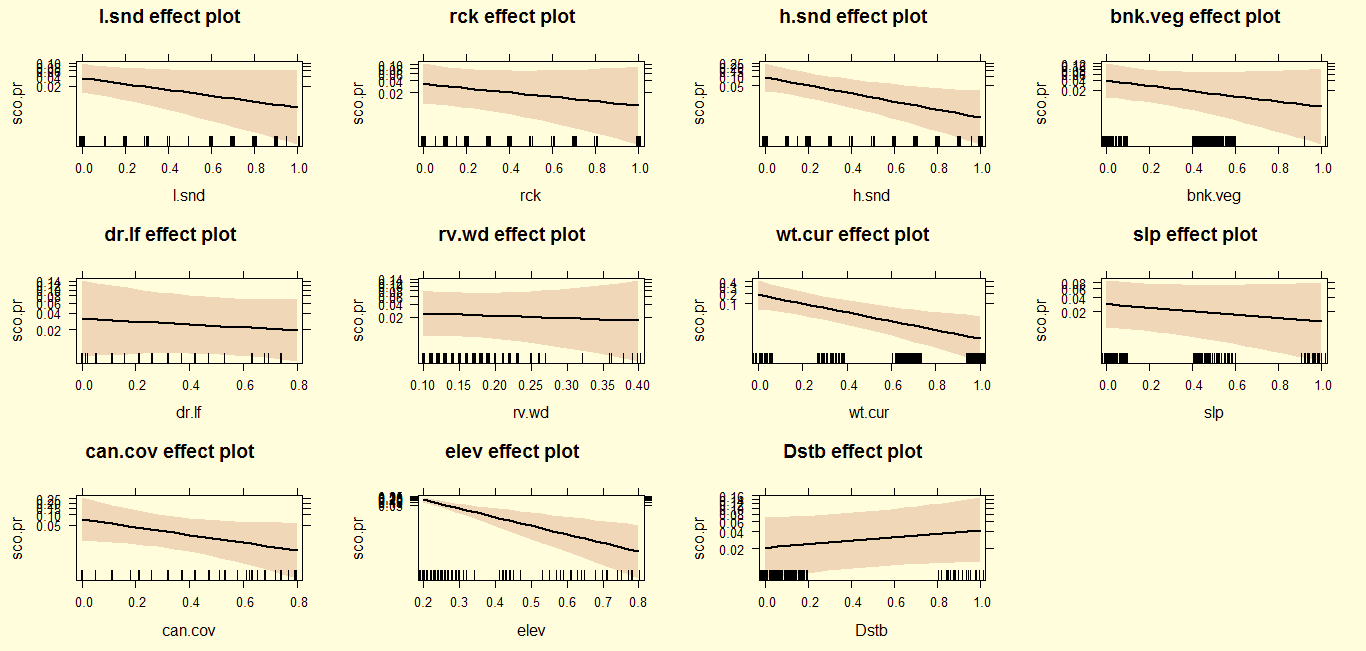
Otter spraint occurrence varied with water current (i.e. fast, slow and stagnant), slow water current yields less spraints. No spraints were recorded at plots where mesquite had invaded (>89%).Spraints were most frequently recorded at plots with minimal shoreline vegetation (<48%).Although, more spraints (mean ± SE 0.57 ± 0.21) were recorded from plots with the presence of associated fauna (i.e. mugger crocodile Crocodilus pardus). Mostly otter spraints (0.37 ± 0.04) were recorded from plots that had loose sand and low disturbance.
DISCUSSION
Effective and targeted conservation action requires detailed information about a species, its distribution, and ecology, as well as the distribution of threats that may adversely affect it. Our knowledge of the conservation requirements of otters remains low, and innovative means of gaining rapid insight into their status are needed to identify urgent conservation cases and inform environmental, agricultural, industrial and other development policies with appropriate biodiversity information in a timely manner.
Otters are excellent indicators and ideal symbols for wetland conservation (Gupta et al., 2016). Our detailed study along the river Moyar suggests that smooth-coated otters have very specific habitat preferences - favouring deep and wide stretches of the river, with gently sloping banks, and sandy and low vegetation cover. Conversely, otters in Periyar Tiger Reserve preferred banks that were gradually sloping usually at the mouths of small streams that joined the main lake (Anoop and Hussain, 2004). The smooth-coated otters in the River Moyar avoided shallow, steeper rocky banks. The number of streams joining the river (possibly influencing congregation of fish and vegetation density) was the most important factor in determining habitat selection by otters here. Hussain (1993) found that the distribution of den and prey availability is a pre-requisite for suitable habitat along the Chambal River, India. Melquist and Hornocker (1983) found that differences in habitat use by North American river otters (Lontra canadensis) resulted primarily from differences in habitat composition.
The preferred areas for smooth-coated otters in the River Moyar were characterized by a bank slope of <40°with sparse vegetation cover and high water depth. These areas extend over very small areas (a few meters) but offer excellent foraging ground. Anoop and Hussain (2004) observed that smooth-coated otters preferred low sloping banks (<5◦) with thick vegetation cover and shallow water in Periyar Tiger Reserve. Kruuk (1995) identified that minimizing the energetic expenditure of foraging is most desirable for otters to sustain themselves (optimal foraging), and therefore areas that offer the most dependable and easy food resources are preferred. In the River Moyar, the otters avoided narrow rocky stretches with deep slopes, where the physical constraints of the river led to less food availability and hampered their hunting ability. In the Moyar River, confluences of tributaries were favored by otters. The depth, turbidity and high congregation of fish populations in such areas provide the otters with easy food at the cost of minimal energy (Pinho et al., 2018). Mapping prey availability throughout the river is essential to study food selection by otters. We could not achieve this completely, however, we documented 16 species of fish consumed by otters in River Moyar (KN unpubl. data) and the relative abundance of these species in different parts of the river was obtained from 30 sampling plots.
Kruuk et al. (1989) reported that there was a strong positive association between dens and freshwater; indicating that habitat selection largely depended on the requirements of otters either for food, sprainting plots or freshwater similarly to coastal otters. The otters equally avoided areas with dense vegetation, which offered dry leaf litter cover or invisibility. The importance of vegetation cover has however, been suggested previously for smooth-coated otters by Ruiz-Olmo and Consalbez (1997), Hussain and Choudhury (1995, 1997), for spotted-necked otters (Lutra maculicollis), for Cape clawless otters (Aonyx capensis) by Procter (1963), Rowe-Rowe (1992), and for the Eurasian otter (Lutra lutra) by Macdonald and Mason (1983). Kruuk and Goudswaard (1990), while investigating the reasons for the declining number of otters in Lake Victoria, Africa, described the virtual absence of otters from a section of the lake where the bank-side vegetation was poor.
The availability of temporary dens and resting plots is an important aspect of river otter habitat (Anoop and Hussain, 2004; Brown and Kodric-Brown, 1977). River otters select these plots according to availability and convenience, although spraint plots that provide protection and seclusion are preferred (Melquist and Hornocker, 1983). This was contrary to the observation in the Moyar, where open areas devoid of vegetation were favoured, and suggests that less vegetation cover on the bank could be important to Smooth-coated otters.
Easy access to the holts was also important and otters seemed to avoid longer trips on land, possibly to reduce their chances of encountering dholes (Cuan alpinus), leopards (Panthera pardus) and tigers (Panthera tigris), which were frequently spotted on the River Moyar. The selection of a sandy substrate for grooming could be because it is drier, thus absorbs moisture faster. Otters showed fidelity for grooming and spraint plots, but some plots were used only once or in one season only. Several authors (e.g. Kruuk et al., 1986; Macdonald and Mason, 1983; Hussain and Choudhury, 1997) have reported plot fidelity by other otter species. These plots are re-marked frequently generation after generation (Erlinge, 1967; Kruuk et al., 1986; Macdonald and Mason, 1983; Hussain and Choudhury, 1997). A similar observation was made during this study where grooming and sprainting plots were almost always revisited by otters. It was noted that during heavy rains certain plots got submerged, but once the waters receded, otters again used these plots. Any unrestricted disposal of dam water and rain could increase the water level of Moyar and has the potential to submerge all the traditional basking and grooming plots of the otters. This might result in them spending more time on land in search of new plots, which could pose an additional threat from terrestrial predators.
Human disturbance, in terms of fishing activities, agricultural runoff mixing and presence of anthropogenic activities also often affects otters (Brown and Kodric-Brown, 1977; Hussain and Choudhury, 1997). In Europe and North America, human disturbance may limit the otter populations (Dubuc et al, 1990; Beja, 1992; Newman and Griffin, 1994). This did not; however, seem to affect the otters in the Moyar. Instead, a tolerable association between otters and humans was observed, but during winter they avoided anthropogenic pressures due to heavy anthropogenic activities.
CONSERVATION IMPLICATIONS
The present study identifies key habitats for otter conservation, and the resultant predictive models have considerable practical application to future exercises, such as implementing conservation actions or population enhancement programmes within the Western Ghats regions. This work may also apply elsewhere in Asia. Our models can be used to predict other areas currently occupied by otters that may be suitable for future research. However, extending the models to a dynamic form could distinguish between other areas that the otters could colonize naturally, and those that they would be unlikely to reach. Smooth-coated otters are important components of aquatic ecosystems and the information presented here should improve humanity’s ability to conserve them.
Acknowledgements: We thank our respective institution for supporting our research activities. Special thanks to Tamil Nadu Forests Department for providing necessary permission vide permit number WL5/20861/2015 and Ref. No. 6612/2015M to Narasimmarajan. K. Field Directors (Sathyamangalam Tiger Reserve &Mudumalai Tiger Reserve), District Forest Officer (Nilgiri North Division), and the Forest Range Officers are thanked for kindly supporting the fieldwork. KN deeply acknowledged the support from “Conservation Leadership Programme” “Rufford Foundation” and “Idea Wild” for financial and materials assistance to the first author. This manuscript is also an outcome of the Science Writing Workshop conducted by the ‘Conservation Leadership Programme in 2017, Bengaluru’, India, and benefited immensely from Dr Martin Fisher and Ms. Laura Owens for their comments on the early draft of the manuscript. We are deeply grateful to our forest warriors (anti-poaching watchers) for their help during the field work. We thank our anonymous referees for their constructive comments, which helped us to improve the quality of the manuscript.
REFERENCES
Delibes, A.M.I. (1987). Food habits of the otter (Lutra lutra) in two habitats of the Donana National Park, SW Spain. Journal of Zoology, 211: 399-406.
Arden-Clarke, C.H.G. (1986). Population density, home range and spatial organization of the cape clawless otter (Aonyx capensis)in a marine habitat. Journal of Zoology, 209: 201-211.
Anoop, K.R., Hussain, S.A. (2004). Factors affecting habitat selection by Smooth-coated otters (Lutra perspicillata) in Kerala, India. Journal of Zoology, 263:417-423.
Anoop, K.R., Hussain, SA. (2005). Food and feeding habits of smooth-coated otter (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology, 266: 15-23.
Beja, P.R. (1992). Effects of freshwater availability on the summer distribution of otters (Lutra lutra) on the southwest coast of Portugal. Ecography,15: 273-278.
Bonesi, L., Macdonald, D.W. (2004). Evaluation of sign surveys as a way to estimate the relative abundance of American mink (Mustela vison). Journal of Zoology, 262: 65-72.
Brown, L.H., Kodric-Brown, A. (1977). Turnover rates in insular biogeography: effect of immigration on extinction. Ecology, 58: 445-449.
Bright, P.W. (2000). Lessons from lean beasts: conservation biology of the mustelids. Mammal Review, 30: 217-226.
Brzezinski, M., Jedrzejewska, B. (1993). Diet of otters (Lutra lutra) inhabiting small rivers in the Bialowieza National Park, Eastern Poland. Journal of Zoology, 230: 495-501.
Champion, H.G., Seth, S.K. (1962). A revised survey of the forest types of India. Manager of Publications. Delhi. 404pp.
Dinerstein, E. (2015). Guiding agricultural expansion to spare tropical forests: agricultural expansion in the tropics. Conservation Letter. 8: 262-271.
Dubuc, L.J. Krhon, W.B., Owen, R.B. (1990). Predicting occurrence of river otters by habitat on Mount Desert Island Maine. Journal of Wildlife Management, 54: 594-599.
Erlinge, S. (1967). Home range of the otter (Lutra lutra) in Southern Sweden. Oikos, 18: 186-209.
Erlinge, S. (1968). Food habits of the otter (Lutra lutra). Oikos, 19: 259-270.
Gupta, N. Johnson, J.A., Sivakumar, K., Mathur, V.B. (2016). The perilous voyage of Indian Himalayan ‘ambassadors’ amidst anthropogenic pressures and changing climatic variables. IUCN Otter Specialist Group Bulletin, 33(1): 33-36.
Hussain, S.A. (1993). Aspect of the ecology of Smooth-coated otter in National Chambal Sanctuary. PhD thesis. Centre for Wildlife and Ornithology, Aligarh Muslim University, India. 206pp.
Hussain, S.A. (1996). Group size, group structure and breeding in smooth-coated otter Lutra perspicillata Geoffroy (Carnivora, Mustelidae) in National Chambal Sanctuary, India. Mammalia, 60 (2): 289-297.
Hussain, S.A. (1998). Conservation status of otters in the Terai and Lower Himalayas of Uttar Pradesh, India. pp. 1-13. In: Proceedings of the VII International Otter Symposium, March 13-19, 1998, Trebon, Czech Republic.
Hussain, S.A., Choudhury, B.C. (1995). Seasonal movement, home range and habitat use by Smooth-coated otters Lutra perspicillata in National Chambal Sanctuary, India. pp. 45-55. In: Proceedings of VI. International Otter Colloquium, Pietermaritzburg. Reuther, C., Rowe-Rowe, D. (Eds.). Aktion Fischofterschutz Hankensbüttel.
Hussain, S.A., Choudhury, B.C. (1997). Status and distribution of Smooth-coated otters Lutra perspicillatain National Chambal Sanctuary. Biological Conservation, 80: 199-206.
Hussain, S.A. (2008). Otter conservation in India. Envis Bulletin - Wildlife and Protected Areas. 2(2): 92-97.
Hussain, S.A., De Silva, P.K., Feeroz, M.M. (2008). Lutrogale perspicillata. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. [www.iucnredlist.org]
Kruuk, H. (1995). Wild otters - Predation and populations. Oxford University Press, 287pp.
Kruuk, H., Goudswaard, P.C. (1990). Effects of changes in fish populations in Lake Victoria on the food of otters (Lutra maculicollis and Aonyx capensis). African Journal of Ecology, 28: 322-329.
Kruuk, H., Moorhouse, A., Conroy, J.W.H., Durbin, L. and Frears, S. (1989). An estimate of numbers and habitat preferences of otters Lutra lutra in Shetland, UK. Biological Conservation, 49(4): 241-254
Kruuk, H., Conroy, J.W.H., Glimmerveen, U., Ouwerkerk, E. (1986). The use of spraints to survey populations of otters (Lutra lutra). Biological Conservation,35: 187-194.
Kumar, S., Mathur, M. (2014). Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands. Tropical Ecology, 55: 33-46.
MacKenzie, D.I., Nichols, J.D., Lachman, G.B., Droege, S., Andrew Royle, J., Langtimm, C.A. (2002). Estimating plot occurrence rates when detection probabilities are less than one. Ecology, 83, 2248-2255.
Macdonald, S.M., Mason, C.F. (1983). Some factors influencing the distribution of otters (Lutra lutra). Mammal Review,13: 1-10.
Medina, G. (1996). Conservation and status of Lutra provocax in Chile. Pacific Conservation Biology, 2: 414-419.
Melquist, W. E., and Hornocker, M. G. (1983). Ecology of river otters in west central Idaho. Wildlife Monographs 83:1-60.
Narasimmarajan, K., Gopal, A., Palanivel, S., Mathai, M.T. (2018). Status of mugger crocodiles (Crocodylus palustris) in River Moyar, Southern India. Cobra. Volume XII (2): 1- 9.
Nawab, A., Hussain, S.A. (2012a). Prey selection by Smooth-coated otter (Lutrogale perspicillata) in response to variation in fish abundance in Upper Gangetic Plains, India. Mammalia,76: 57-65.
Nawab, A., Hussain, S.A. (2012b). Factors affecting the occurrence of smooth-coated otter in aquatic systems of the Upper Gangetic Plains, India: Smooth-Coated Otter in Upper Gangetic Upper Plains. Aquatic Conservation: Marine and Freshwater Ecosystem,22: 616-625.
Newman, D.G., Griffin, R. (1994). Wetland use by river otters in Massachusetts. Journal of Wildlife Management, 58: 18-23.
Procter, J. (1963). A contribution to the natural history of the spotted necked otter (Lutra maculicollis) in Tanganyika. East African Wildlife Journal, 1: 93-113.
Puyravaud, J.P., Davidar, P. (2013). The Nilgiris Biosphere Reserve: an unrealized vision for conservation. Tropical Conservation Science, 6: 468-476.
Raj, B.S. (1941). Dams and fisheries: Mettur and its lessons for India. Proceedings of the Indian Academics of Science,14 (5): 341-358.
Rowe-Rowe, D.T. (1992). Survey of South African otters in a freshwater habitat, using sign.South African Journal of Wildlife Research,22(2): 49-55.
Ruiz-Olmo, J., Gonsalbez, J. (1997). Observations on the spraintingbehaviour of the otter (Lutra lutra L. 1758) in the North East Spain. Acta Theriologica, 42: 259-270.
Satyanarayana, D. (1997). Studies on smooth-coated otter (Lutrogale perspicillata Geffroy) in two ecologically different habitats in south India with special emphasis on its conservation. PhD thesis, Osmania University.
Pinho, F.F., Ferreira, G.B., Barata, I.M. (2018). Feeding ecology and spraint deposition plots of the Neotropical Otter (Lontra longicaudis) at Cavernas do Peruaçu National Park, Brazil. IUCN Otter Spec. Group Bull., 35: 11-21
Van Emden, H.F. (2008). Statistics for terrified biologists. Blackwell Pub.
White, P.C.L. McCleana, C.J., Woodroffe, G.L. (2003). Factors affecting the success of an otter (Lutra lutra) reinforcement programme, as identified by post-translocation monitoring. Biological Conservation, 112: 363-371.
Zar, J.H. (1984). Biostatistical analysis. Ind. edn. Prentice-Hall Inc., New Jersey. 718pp.
Resumé: Étude de la Présence et Modélisation de l’Utilisation des Ressources des Loutres à Pelage Lisse Lutrogale perspicillata Geoffroy (Carnivores, Mustélidés) le long de la Rivière Moyar dans le « Hotspot » de la Biodiversité des Ghats Occidentales
Comprendre les facteurs affectant la présence et les besoins en matière d’habitat des espèces menacées est fondamental pour leur protection. Les modèles d’occurrence et d’utilisation des ressources par la loutre à pelage lisse, vulnérable, ont été examinés entre 2015 et 2017 par échantillonnage des épreintes de loutres, des pistes, des tanières et des sites de toilettage le long de la rivière Moyar dans le «Hotspot» de la biodiversité des Ghats occidentaux en Inde. Un modèle basé sur l’occurrence a été utilisé pour déterminer l’influence des co-variables environnementales sur la détectabilité de la loutre. Des informations sur les paramètres environnementaux ont été enregistrées, tous les 400 mètres, le long des berges durant les saisons d’après mousson, d’hiver et d’été. La présence de loutres à pelage lisse était élevée avant l’été, mais a considérablement diminué après la mousson lorsque l’élévation du niveau d’eau réduit la détectabilité des loutres en éliminant les indices de présence. L’occurrence des loutres et les modèles d’utilisation des ressources ont été influencés par le substrat de la rivière, les caractéristiques de l’habitat et des berges, et les différents milieux forestiers. Les loutres préfèrent des rivières plus larges, mais évitent les zones rocheuses avec une faible profondeur d’eau. Les modèles d’utilisation des ressources ont été déterminés par les caractéristiques de l’habitat et les perturbations au cours des trois saisons. Diverses formes de perturbations ont nui à la présence de la loutre. La restauration des habitats dégradés améliore les perspectives de conservation des loutres à long terme. Les plans régionaux de conservation de la loutre doivent être spécifiques à l’espèce pour aider au maintien de l’équilibre écologique de l’écosystème rivière Moyar.
Revenez au dessus
Resumen: Evaluación de la Ocurrencia y Patrón de Uso de Recursos de la Nutria Lisa Lutrogale perspicillata Geofroy (Carnivora, Mustelidae) en el Río Moyar, Hotspot de Biodiversidad de los Ghats Occidentales
Entender los factores que afectan la presencia y los requerimientos de hábitat de las especies amenazadas, es fundamental para su conservación. Examinamos los patrones de ocurrencia y de uso de recursos por parte de la nutria lisa, muestreando el Río Moyar en el hotspot de biodiversidad de los Ghats Occidentales, India, entre 2015 y 2017, en busca de fecas, huellas, madrigueras y sitios de acicalamiento. Utilizamos un marco basado en la ocurrencia, para determinar la influencia de las covariables ambientales en la detectabilidad de las nutrias. La información sobre los parámetros ambientales fue registrada cada 400 metros a lo largo de las barrancas del río en las estaciones post-monzones, invierno, y verano. La ocurrencia de nutria lisa fue alta antes del verano pero disminuyó drásticamente después de los monzones, cuando los niveles aumentados de agua reducen la detectabilidad de las nutrias lavando los signos de su presencia. Los patrones de ocurrencia y uso de de recursos por las nutrias estuvieron influenciados por el sustrato del río, las características del hábitat, los rasgos de la barranca del río y los tipos forestales. Las nutrias prefieren mayor ancho del río, pero evitaron las áreas rocosas con agua poco profunda. Los patrones de uso de recursos estuvieron determinados por los rasgos del hábitat y los disturbios, en todas las estaciones. Varias formas de disturbio afectaron adversamente la ocurrencia de nutrias. La restauración de hábitats degradados es necesaria para mejorar las perspectivas de conservación de largo plazo de las nutrias. Se necesita que los planes regionales de conservación de nutrias sean especie-específicos, para ayudar a mantener el balance ecológico en el ecosistema del Río Moyar.
Vuelva a la tapa