IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Citation: Pianzin, A., Wong, A. and Bernard, H. (2021). Riparian Reserves serve as a Critical Refuge for Asian Otters (Aonyx cinereus and Lutrogale perspicillata) in Oil Palm Dominated Landscapes of Sabah, Malaysian Borneo. IUCN Otter Spec. Group Bull. 38 (3): 133 - 154
Riparian Reserves serve as a Critical Refuge for Asian Otters (Aonyx cinereus and Lutrogale perspicillata) in Oil Palm Dominated Landscapes of Sabah, Malaysian Borneo
Annabel Pianzin1,2*, Anna Wong1,2 and Henry Bernard1
1Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia
2Malaysian Nature Society, JKR 641 Jalan Kelantan, Bukit Persekutuan, 50480 Kuala Lumpur, Federal Territory of Kuala Lumpur, Malaysia
* Corresponding Author Email: criikey.mate@yahoo.com
(Received 4th May 2020, accepted 4th November 2020)
Abstract: We determined the occupancy of otter species and assessed several habitat features influencing their occurrence in four different land-use types: continuous logged forests (CF), heavily degraded forest (DF), riparian reserves within oil palm plantation (RR), and oil palm plantations without riparian reserves (OP). Our aim was to ascertain the usefulness of retaining riparian reserve in oil palm dominated landscape for otter conservation. This study was conducted in the Malaysian state of Sabah, northern part of Borneo. We surveyed 36 stream sub-transects across all of the different land-use types and detected otter presence based on their tracks and spraints. Overall, two out of the four otter species found in Sabah were detected within the surveyed areas, i.e., the Asian Small-clawed Otter, A. cinereus and Smooth-coated Otter, L. perspicillata. Streams in agricultural sites were found to have significantly higher otter occupancy compared to forested areas: RR (psi = 0.97), OP (0.83), DF (0.44), and CF (0.37). Using Generalised Linear Modelling (GLM), we identified that otter occupancy in oil palm landscapes was positively influenced by the availability of large trees and other vegetation along the banks. Deeper streams were also more preferred by otters. Interestingly, streams in oil palm plantations located nearer to human settlements recorded higher detection of otter signs. In general, this study suggests that streams in oil palm plantation with riparian vegetation are useful habitat for otter species. Hence, retaining riparian reserves within oil palm plantations is a useful management strategy to improve biodiversity conservation in an agricultural landscape.
Keywords: riparian reserves, oil palm, land-use changes, otter, occupancy, sustainable oil palm plantation.
INTRODUCTION
Land-use changes particularly the conversion of tropical forests into agricultural plantations, i.e. oil palm, have been known to affect riparian habitats and have a strong negative influence on the freshwater ecosystem, due to the destruction of distinct vegetation community and structure of the riparian zones affecting every trophic level (Feld et al., 2011; Thomas et al., 1979). Habitat modifications as a result of agricultural plantation development and logging directly influence the semi-aquatic otters (Order: Carnivora; Family: Mustelidae) since these activities lead to changes in the structure of their habitats on land from spatially complex into a much simpler structure (Danielsen et al., 2009; Foster et al., 2011). In turn, this degrades stream quality and subsequently affects many aquatic organisms and other aquatic resources that the otters depend on for their survival (Bedford, 2009; Struebig et al., 2008).
Previous studies have shown that biodiversity within cultivated lands could be sustained to some extent through the development and management of riparian reserves (Bernard et al., 2014; Giam et al., 2015; Gray et al., 2014; Gray et al., 2015; Luke et al., 2017a; Mitchell et al., 2018; Peel, 2018; Seaman et al., 2019; Sekercioglu et al., 2015; Wilkinson, 2018). Riparian reserves themselves are not only critical habitats for numerous species of animals, but at the same time they maintain critical ecological functions such as filtering agricultural chemicals and protect against soil erosion (Lamb et al., 2006; Luke et al., 2017b; Sabo et al., 2005; Turner et al., 2008). The threats affecting riparian carnivores in highly modified tropical landscapes are poorly understood (Laws, 2016), and there is limited information on habitat requirements of semi-aquatic vertebrates, such as the otters, especially in oil palm dominated landscapes. Regarded as an important indicator of the wetland environment, otters are sensitive to changes in the quality of their preferred habitats including both the aquatic environment and the associated riparian habitats (Shenoy et al., 2003). In agricultural landscapes across Asia, past studies revealed a relationship exist between otter presence and certain environmental features such as the river bank vegetation, canopy cover, substrate type, prey availability, river depth and width, disturbance intensity, and distance from human settlements (Aadrean and Usio, 2017; Laws, 2016; Pillai, 2015; Prakash et al., 2012; Prakash et al., 2014).
The preservation of native vegetation bordering water bodies has been widely adopted as a useful mitigation strategy in reducing the negative impacts of land-use changes in monoculture agriculture. In fact, it is one of the criteria of certification for sustainable palm oil production under the Roundtable on Sustainable Palm Oil scheme (RSPO, 2013). The legislation of Sabah presently requires riparian buffers with a minimum of 20 m and up to 30 m wide to be retained on all streams with a width of 3 m and 10 m, respectively (EPD, 2011; SWRE, 1998). Its purpose is to preserve water volume and flow, prevent degradation of water quality, and subsequently destruction to the aquatic ecosystem (SWRE, 1998). With proper management and planning of habitats in modified agriculture landscapes, they have a potential be a vital habitat for biodiversity in their own merit (Mendenhall et al., 2012). The establishment of sustainable oil palm plantation might be able to offer protection on otter habitats by ensuring suitable buffer widths along water bodies to be preserved, and by practicing good management for handling of wastes, protection of slopes, and agricultural conversion in degraded lands instead of forests.
In the present study, we aim to ascertain the value of retaining riparian reserves in oil palm plantation for otter conservation. We compare otter occupancy between riparian reserves in continuous logged and heavily degraded forested areas, and in oil palm plantations (with and without riparian reserves) located in Sabah, northern part of Malaysian Borneo. We investigate several environmental variables that may influence the occurrence of otters in both forested areas and agricultural sites. We hypothesized that otter occupancy would be higher in forested habitats compared to in oil palm plantations. Whereas within the oil palm plantations, we expected that otter occupancy would be higher in the oil palm with riparian reserves compared to oil palm without the riparian reserves.
METHODOLOGYStudy Area
Specifically, the present study was conducted in and around the ‘Stability of Altered Forest Ecosystems’ (SAFE) Project (117.50oN, 4.6oE) area in Sabah, Malaysian Borneo (Figure 1). The study area is approximately 80,000 ha comprising twice-logged lowland dipterocarp rainforest, acacia plantations, and oil palm plantations, the latter was established between 1999 and 2014 (Gray et al., 2015; Mitchell et al., 2018; Wilkinson et al., 2020). The area has a high annual rainfall of 2,925 mm, 2017–2018 (Malaysian Meteorological Department, 2019). There is little climate seasonality in the study area where drought only occurred during major El Niño Southern Oscillation years (Luke et al., 2017b).
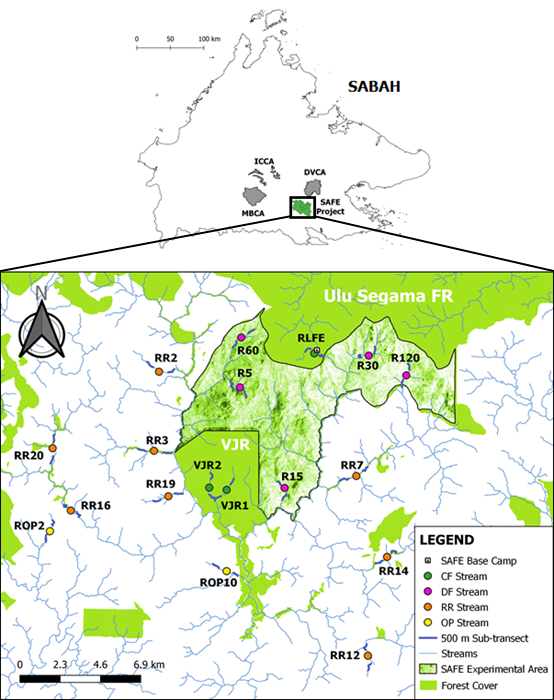
The SAFE Project is a large-scale, long-term ecological experiment to explore the effect of forest degradation and fragmentation and oil palm plantation development on the biodiversity and ecosystem functions (Ewers et al., 2011). The SAFE Project experimental area was part of an area that has been gazetted for the conversion to agricultural plantation, mainly oil palm, by the Sabah state government since more than 20 years. At the time of the present study, the vegetation in the study area comprised mainly of heavily degraded forests which have been selectively logged multiple times (Ewers et al., 2011). Despite the high level of habitat degradation, the richness of animal community in this area was very high (Bernard et al., 2016; Wearn et al., 2017). Two of the common otter species of Sabah (A. cinereus and L. perspicillata) have been reported to occur in streams of the study area (Laws, 2016).
We selected streams in this study to represent the norm of the main habitat changes found within this region of Sabah and consisted four general categories of land-use types: continuous logged forest (CF), heavily degraded forests within heavily logged area planned for oil palm conversion (DF), forested riparian reserves in oil palm plantation (RR), and oil palm without riparian reserves (OP). (Fig 2)
Continuous Logged Forests (CF)
This land-use type was represented by three streams located immediately outside the SAFE experimental area. Two streams (VJR1 and VJR2) were located in old-growth forest sites within the Brantian-Tantulit Virgin Jungle Reserve (VJR) and one stream (RLFE) in the continuous forest adjoining the Ulu Segama Forest Reserve. The 2,200 ha VJR area underwent minimal illegal logging around the edges for road construction in the 1970s and 1990s but not to the extent of commercial selective logging (Ewers et al., 2011; Luke et al., 2017b; Struebig et al., 2013). Most of the old-growth forest characteristics in this site remain undisturbed (Struebig et al., 2013). RLFE stream is located within a twice-logged forest where it was selectively harvested in the 1970s and late 1990s to early 2000s, although 71% of its forest cover remains intact (Struebig et al., 2013).
Heavily Degraded Forests (DF)
The heavily degraded forests were represented by five streams (R5, R15, R30, R60, and R120). The experimental area where all heavily degraded forest streams were located consisted of 7,200 ha of lowland dipterocarp rainforest. It is situated neighbouring the VJR and to a large forested area (greater than 1 million ha) encompassing three large conservation areas: Danum Valley (DVCA), Maliau Basin (MBCA), and Imbak Canyon (ICCA), where all three have never been logged (Ewers et al., 2011). This area has undergone multiple rounds of selective logging in the 1970s, followed by multiple further rounds from the late 1990s to 2000s. All commercially valuable trees were removed between 2013 and 2016 (Seaman et al., 2019). The forest remnants within the experimental area only hold a small number of mature trees; however, some areas are less disrupted and are protected by law (Struebig et al., 2013). The area is extremely heterogeneous with forest patches of closed-canopy intermixed with regrowth, gaps, and roads (Luke et al., 2017b).
Riparian Reserves within Oil Palm Plantation (RR)
The riparian reserves in oil palm plantations were represented by eight streams (RR2, RR3, RR7, RR12, RR14, RR16, RR19, and RR20) that were retained within mature oil palm plantations planted between 1999 and 2014 (Mitchell et al., 2018; Wilkinson et al., 2020). The width of the riparian reserves along each of the eight streams was approximately 50 m on average with a range between 10 to 470 m (Mitchell et al., 2018).
Oil Palm Plantation without Riparian Reserves (OP)
The oil palm plantations without riparian reserves (ROP2 and ROP10) were represented by two streams. The oil palm estates in this study site were planted in 2009 and were located immediately outside the experimental area but adjacent to the streams selected in the oil palm with riparian reserves explained above.

Otter Survey Methods
We surveyed otter species in a total of 18 riparian sites (Fig 1), each of which were divided into 2 independent sub-transects. Therefore, the overall number of sub-transects surveyed for otter was 36 sub-transects. Each riparian site consisted of two km line transect divided into two 500 m sub-transects separated by a one km gap. This was to minimise the possibility of counting the same individual in multiple segments of the same riparian sampling site (Laws, 2016). Each sub-transects were further divided into 100 m segments to facilitate systematic data recording in the field. Site visitations for otter survey were done at random with an interval between two days to three months apart between sites visitations. The sampling sessions were completed for the first 14 streams (VJR1, RLFE, R15, R30, RR2, RR3, RR7, RR12, RR14, RR16, RR19, RR20, ROP2, and ROP10) in July-August 2017, September-October 2017, November 2017, and March 2018. The remaining four streams (VJR2, R5, R60, and R120) were sampled in May-June 2018.
During the surveys, we conducted searches for otter signs on foot on both sides of the stream banks (up to 13 m from stream edge), as well as on the rocks and boulders in the streams. A minimum of two observers was involved in the survey, i.e., one at each stream bank. The survey was conducted in the mornings to afternoons (8.30 - 14:00 hrs.) and concluded between late mornings to late afternoons (10:00 - 16:30 hrs.), depending on weather condition and proximity of the SAFE Project base camp to streams. Detected otter signs were recorded throughout the 500 m sub-transects. Indirect signs were comprised of spraints, tracks, and grooming areas. Direct observations of otters were recorded when they were encountered. Otter species identity from indirect signs were categorised as belonging to either species based on specific features.
Smaller tracks with partial webbing and without prominent claws was classified as A. cinereus, whereas larger tracks with full webbing and prominent claws was classified a L. perspicillata (Francis, 2008; Hussain, 2016; Wai, 2018). Likewise, spraints were categorised as belonging to either one of the species based on undigested prey content, appearance, and habitat. Spraints with higher content of crustaceans were classified as A. cinereus and spraints with higher fish content was classified as L. perspicillata (Foster-Turley, 1992; Hussain, 2016; Wai, 2018). Uncertain spraints were identified with the aid of an experienced otter researcher. During every survey, the occurrence of otters based on indirect and direct signs was documented as ‘1’ to indicate otter presence and ‘0’ for absence. The location coordinates of the otter signs were recorded using a handheld GPS.
Habitat characterisation
To predict the potential habitat features influencing otter occurrence, we measured a total of 13 environmental variables (Table 1) on the stream and stream bank at regular intervals along each 100 m segment within the 500 m sub-transects at all riparian sites. Vegetation plots of 5 x 5 m were established at each bank to determine bankside vegetation condition both at canopy and ground level. In addition, 3 disturbance variables were also recorded at each 500 m sub-transect to observe their influence on otter occurrence.
Data Analysis
The habitat variables measured were grouped by land-use types. Bank substrates were converted into proportion in sub-transects as soil/sand, rocks, or a combination of both. Data containing proportion and percentages were transformed using the ‘arcsine’ transformation for data normalization and to stabilize the variance. Average of averages of habitat variables were used to approximate habitat characteristics as mean ± SE for each land-use type. All analyses were performed using RStudio version 1.1.463 (R Core Team, 2019). The occupancy of otters dependent on land-use types was modelled with the package ‘unmarked’ (Fiske and Chandler, 2011) for each land use types, by individual otter species and combined. The smallest independent sampling unit was the 500 m sub-transects, of which only signs detected within them were included in the analyses. Site occupancy and detection probability of an individual were calculated based on Mackenzie et al. (2006), which accounts for the likelihood of an individual inhabiting the area and being detected during a survey. Detection histories of otters in each land-use types were based on whether otters or their signs were detected in each temporal replicate. Single-season, single-species occupancy modelling was used, and analysis was run when occupancy (psi) and detection probability (p) were held at constant. Further analysis was conducted to investigate the effect of land-use types on psi when p was held constant using the “aictab” function (“AICcmodavg” package, Mazerolle, 2020), and the best model, i.e., with delta AICc ≤ 2 was selected as the most parsimonious model explaining otter occupancy in the study site.
Stream and bank variables that were highly correlated with one or more variables (correlation coef. r > 0.50) were eliminated based on Pearson’s Correlation Coefficient. The retained habitat variables were used as site covariates in the Generalised Linear Models (GLMs) to model otter occurrence, by taking the presence and absence of spraints and tracks as the response variable with binomial distribution family and logit link function. All predictor variables were standardised by using the "scale" function (“base” package, R Development Core Team, 2019). Next, the “dredge” function under the “MuMIn” package (Barton, 2016) was used on the global model to select the best set of candidate models and evaluated the relative importance of variables based on delta (Δ) AICc (Burnham and Anderson, 2002). The second-order Akaike information criterion (AICc) was used due to the small sample size of this study. The “dredge” function was performed to generate all possible combination of models by taking into account the set of predictor variables in the global model. The 95% confidence interval with averaged coefficients and the relative importance for each predictor variables from the selected set of candidate models with ΔAICc ≤ 2 was calculated by using the “model.avg” function (“MuMIn” package, Barton, 2020). Models were compared by ΔAICC and Akaike weight (wi) to assess model fit, where the model selection was prioritised for each species and combined with a minimum of ΔAICc ≤ 2, and model with wi ≤ 0.05 were dismissed (Burnham and Anderson 2002).
RESULTS
Visual signs survey through the presence of spraints and tracks confirmed the presence of two otter species, A. cinereus and L. perspicillata, in 13 out of the 18 streams surveyed (Figure 3). A total of 87 otter signs were detected in 36 sub-transects during the study, where 34 signs belonged to L. perspicillata (39.0%) and 53 signs to A. cinereus (60.9%). Otter signs were mainly found within RR streams (49 signs; 56.32%), followed by DF (23; 26.44%), OP (8; 9.19%), and CF (7; 8.05%). The average of signs detection in the 500 m sub-transects per land-use types were 3.06, 2.03, 2.00, and 1.17, respectively. Out of the otter signs detected, 52 (59.77%) were footprints and 35 (40.23%) were spraints. Overall, the total accumulated sampling effort was over 72 days of survey occasions was 205 hours and the cumulative distance travelled in all survey transects combined across 18 streams was 144 km.

Otter Occupancy
Overall, otter signs were detected in 23 of the 36 sub-transects surveyed within four land-use types resulting in a naïve occupancy of 0.64. When modelled occupancy (psi) and detection probability (p) were constant across land-use types, otter occupancy at the landscape level was 0.71 ± 0.10 (SE), with an estimated overall p of 0.44 ± 0.06. Signs of A. cinereus were detected in 17 of the 36 sub-transects surveyed, which resulted in a naïve occupancy of 0.47. The psi of this species was 0.59 ± 0.12, and p was 0.33 ± 0.07. While the signs of L. perspicillata were detected in 13 of the 36 sub-transects surveyed, resulted in a naïve occupancy of 0.36. The psi of this species was 0.49 ± 0.14, and p was 0.28 ± 0.08. Because the main objective of this study was to investigate the effect of land-use on otter presence, occupancy was assessed by species and combined, and separately for each land-use types – continuous logged forest (CF), heavily degraded forest (DF), oil palm plantation with riparian reserves (RR), and without riparian reserves (OP).
By taking into account the type of land-use changes across the landscape, A. cinereus was detected the highest in RR, followed by CF, DF, and OP. Signs were detected in 11 out of the 18 sub-transects (naïve occupancy = 0.69) in RR, 2 out of the 6 (0.33) sub-transects in CF, 3 out of the 10 (0.30) sub-transects in DF, and 1 out of the 4 (0.25) sub-transects in OP. When corrected for detection probability, psi in the four land-use types were marginally higher compared to naïve estimates at 0.86 ± 0.17, 0.42 ± 0.24, 0.38 ± 0.19, and 0.31 ± 0.27, respectively. The p was comparable in both CF and DF (0.17 ± 0.11, 0.18 ± 0.09, respectively), and was remarkably higher in RR (0.38 ± 0.08) and lower in OP (0.08 ± 0.08).
Meanwhile, L. perspicillata was detected the highest in both RR and OP, followed by DF and CF. Signs were detected in 8 out of the 16 (0.50) sub-transects in RR, 2 out of the 4 (0.50) sub-transects in OP, 3 out of the 10 (0.30) sub-transects in DF, and 0 out of the 6 (0.00) sub-transects in CF. Since there was no detection in CF for this species, the psi and p were excluded from the following details and the effect of land-use types on otter occupancy (Table 2). The psi was slightly higher than naïve occupancy at 0.68 ± 0.20, 0.68 ± 0.36, and 0.41 ± 0.21, respectively. The p for each land-use type was 0.27 ± 0.09, 0.30 ± 0.17, and 0.34 ± 0.20, respectively.
The effect on land-use types was analysed in regards to psi when p was held at constant, and the best model with ΔAICc ≤ 2 was selected as the best model explaining otter occupancy in the study site is shown in Table 3. For A. cinereus, p(.), psi(RR) emerged as the best predictor for land-use type whereas for L. perspicillata, the null model, p(.), psi(DF), and p(.), psi(RR) were the best predictor explaining occupancy of the species in the study area.
Otter Presence Identified Important Habitat Features in Oil Palm Dominated Landscapes
Generalized linear models (GLMs) were used to model the occurrence of otters by species and combined as a response variable on habitat features which included stream and banks variables, as well as disturbances. Non-correlated variables were selected based on a correlation matrix and were then used to create the global model. The variables selected were stream width and depth, altitude, stream edge, undergrowth cover, bank substrate based on the proportion of soil, or combination of soil and rocks, number of trees, bank tree GBH, distance to human settlements, and disturbance history. The habitat variables for the global model remained unchanged for all analyses. Since otters are known to travel over long distances due to the linear nature of their habitat and the proximity between forested areas and oil palm plantation taken into consideration, the analyses were run at the overall landscape level.
Both species
The final model selected – Otter presence ~ Bank tree GBH + Proportion of bank with soil and rocks combination + Stream depth + Distance to human settlements + Bank undergrowth cover
In the model consisting of both species occurring in the study area, the final model selected based on AICC scores comprised of bank tree GBH, the proportion of bank with soil and rocks combination, stream depth, distance to human settlements, and bank undergrowth cover (AICC=38.06, ΔAICC=0.00). Bank tree GBH had a positive relationship with the presence of otters and was significant at 0.05. Three variables, namely the proportion of bank with the combination of soil and rocks, stream depth, and bank undergrowth cover, had positive relationships to otter presence and were significant at 0.01. The distance to human settlements had a negative relationship and was significant at 0.01 (Table 5).
The Asian-small clawed otter (Aonyx cinereus)
Final model selected – Otter presence ~ Altitude + Proportion of bank with soil + Distance to human settlements
For this species, the best-fit model consisted of three habitat variables which were altitude, the proportion of bank with soil, and distance to human settlements (AICC=42.74, ΔAICC=0.00). There was a positive relationship to altitude and was highly significant at 0.001 level. The proportion of bank with soil showed a positive relationship and was significant at 0.05. The distance between streams to human settlement had a negative relationship and was significant at 0.01 (Table 6).
The smooth-coated otter (Lutrogale perspicillata)
Final model selected – Otter presence ~ Stream depth + Number of trees + Bank undergrowth cover + Stream width
For this species, the final model selected consisted of four habitat variables which were stream depth, number of trees, bank undergrowth cover, and steam width (AICC=43.96, ΔAICC=0.00). Stream depth had a positive relationship with the response variable and was significant at 0.01. There was a positive relationship with stream width and was significant at 0.05. The non-significant variables included in the final model were the number of trees and bank undergrowth cover (Table 7).
DISCUSSION
The occupancy of otters in this study varied across land-use types in a sustainably-managed oil palm dominated landscapes of Sabah. Streams located in agricultural sites: with riparian reserves (psi = 0.97) and without riparian reserves (0.83) have higher otter occupancy compared to forested areas: heavily degraded forest (0.44) and continuous logged forest (0.37). This indicates that they can persevere in a variety of habitat quality and that riparian reserves serve as crucial refuges for otters even in such modified landscapes. The result obtained contrasted with the initial hypothesis tested of sites in forested areas having higher otter occupancy compared to oil palm plantations. Despite that, it corresponds to several studies where otter occupancy was found to be somewhat insensitive towards human-modified landscapes for A. cinereus and L. perspicillata (Cho et al., 2009; Foster-Turley, 1992; Jo et al., 2017; Kamjing et al., 2017; Laws, 2016; Ottino and Giller, 2004; Pillai, 2015; Prakash et al., 2012; Prakash et al., 2014). When occupancy was observed by species, both A. cinereus and L. perspicillata have the highest occupancy in riparian reserves at 0.86 and 0.68, respectively. With consistent finding of high otter occupancy in riparian reserves on multiple occasions in various streams, it supports the previous claim that otters are residents in reserves instead of transient individuals or groups (Laws, 2016).
The main reason why otters can persist in a wide range of habitat quality is due to the linear nature of their habitats, which permits them to travel over long distances to meet their demands (Prakash et al., 2012). They may also have core areas spread across multiple land-use types because of specific habitat characteristics preferred. Some preferred habitat characteristics include dense bankside vegetation for holt placement (Anoop and Hussain, 2004; Prakash et al., 2014; Shenoy et al., 2006), and areas with sparse to no vegetation, low canopy cover, and loose sand for basking and grooming sites (Anoop and Hussain, 2004; Shenoy et al., 2006). Areas containing loosely packed sand with a small amount of rock but lacking in canopy cover, dense vegetation and stony or gravely substrate were chosen as sprainting sites (Shenoy et al., 2006). The proximity between plantations with and without riparian reserves suggests that higher otter occupancy in the latter might because of suitable habitat features available in mature riparian reserves neighbouring agricultural sites. These reserves contain ample vegetation cover, suitable streamline substratum, and various prey species important for otter activities such as denning, grooming, sprainting, foraging, and travelling. Without the maintenance of riparian reserves in plantations providing these important habitat features, there is a high chance that otter may not survive in this monoculture environment alone.
Since otters are usually infrequent visitors in extremely small rivers, they are used as feeding grounds or migratory pathway given that they are unable to support sedentary breeding population (Romanowski et al., 2012). This is applicable to streams without riparian reserves in plantations as their sizes were exceptionally smaller compared to any other land-use types. Thus, they cannot sustain otters long term due to their narrow and shallow stream, low canopy cover, and limited vegetation diversity for the security of holt placement. Both heavily degraded forests and riparian reserves recorded the highest detection probability at 0.52 and 0.49, respectively because of sandy banks availability in most streams which permit tracks to be detected and identified without difficulty. Spraints were strongly tied to the presence of rocky substrate detected in continuous logged and heavily degraded forests, as well as riparian reserves. However, most were located outside of the surveyed sub-transects and therefore were excluded from the analyses. Similar to the previous reason, occupancy was lower in continuous logged and heavily degraded forests may be influenced by signs found in the catchments within land-use types but were located outside of the sub-transects. Furthermore, the terrain of streams greatly influenced the detection of otter signs, where streams in agricultural sites were easily traversed compared to forested streams with rugged topography.
Otters can utilise whatever resources available within their habitat due to their opportunistic nature, which has been recorded in terms of prey and habitat availability. Otters commonly respond to the presence of habitat characteristics they use over the presence of anthropogenic structures or activities in an area (Aadrean and Usio, 2017; Gallant et al., 2009; Kamjing et al., 2017; Khoo and Sivasothi, 2018; Shenoy et al., 2003; Theng et al., 2016). Although, not all species of otters have a high resilience towards human-modified landscapes. In Borneo, A. cinereus and L. perspicillata are relatively common where they are often sighted, whereas both the L. lutra and L. sumatrana are rarely seen. This might be because of social otters dominate major habitats, while the two solitary otter species are forced into marginal habitats (Phillipps and Phillipps, 2016). One possible reason why the two rare species were not sighted in the study area was because it is located within a highly disturbed environment. They are known to occur in intact undisturbed or minimally disturbed forests, or in mangroves and swamp areas from recent discoveries which were absent or limited in the surveyed streams.
Since each otter species has different habitat preferences and needs, it is important to identify important habitat features to facilitate protection and conservation on the respective species and their environments. The GLM analysis identified one model that best explains the presence of both otter species at the landscape level which includes bank tree GBH, the proportion of bank with soil and rocks combination, stream depth, distance to human settlements, and bank undergrowth cover which were all significant. Another model which explained the presence of otters equally (ΔAICc ≤ 2) includes all the habitat variables above, with addition to altitude. The ability of observers to identify otter signs are highly influenced by the type of bank substrate which had a positive correlation to presence. The detection of tracks was easier on sand since they imprint well on the substrate, but difficult on rocks or pebbles since signs were normally unclear or do not imprint at all. Both rocky and sandy substrates are suitable mediums for the detection of spraints. Even though higher undergrowth cover is important for otters as a shelter during travel and placement of holt, they impede the detection of signs.
Bank tree GBH and bank undergrowth cover had positive correlations to the presence of otters showing that otters prefer areas with mature vegetation and well-covered banks. Trees that are taller and have bigger diameter provide stream banks with ample canopy cover and smaller, immature tree saplings and shrubs provide undergrowth cover vital for protection against predators. Stream depth too had a positive correlation. Streams within riparian reserves were deeper compared to other land-use types, although the streams in reserves were not deep enough to hinder hunting sessions. Conversely, the distance between streams to human settlements had a negative correlation to otter presence. This indicates that both otter species in the study area are highly adaptable and can tolerate moderate to high level disturbances. As otters need water as foraging and travelling sites, and humans tend to build settlements nearby water sources for their daily needs, both of them will inevitably share the same space. It may cause conflicts to arise when they compete for food resources as they are known as pests to fishermen and pond owners, and as a result are susceptible to being killed (Duplaix and Savage, 2018; Kruuk, 2006; Prakash et al., 2014).
The best-fit model explaining the presence of A. cinereus in oil palm dominated landscapes retained altitude, the proportion of bank with soil, and distance to human settlements, and were all significant. Altitude was positively correlated to otter presence. Although A. cinereus was found in all land-use types, only this species was found to reside in smaller hilly, rocky streams located at higher altitude. The main factor for this occurrence would be the type of main prey species they consume, i.e., crustaceans which can be found in streams with rocky substratum (Foster-Turley, 1992; Kruuk, 2006). The proportion of bank with soil had a positive correlation. Otter detection rate was higher when there was higher proportion of exposed soil. Soil, especially sandy substrate, is known to be an important feature of the otter habitat because it is associated with grooming activity and an important substrate for sprainting site (Anoop and Hussain, 2004; Shenoy et al., 2006). The distance between streams and human settlements had a negative correlation. As mentioned previously, otters usually share space with humans due to the same resources needed for livelihood. However, they will avoid humans by coming out when there are fewer disturbances. Other models that seemed to explain presence of this species equally across the landscape included vegetation variables such as stream edge, bank undergrowth cover, and bank tree GBH. All these are important as security during travelling, resting, and holt placement, as well as to screen disturbances between surrounding land-use and the stream (Prakash et. al., 2014).
The GLM analysis identified one model that best explains the presence of L. perspicillata in the study area. The model retained stream width and depth as significant variables and had positive correlations. Two other non-significant variables included into the final model were number of trees and bank undergrowth cover. L. perspicillata usually inhabit medium to large water bodies containing high fish productivity and tends to hunt for larger preys compared to other otter species sharing the same habitat (Kruuk et al., 1994; Anoop and Hussain, 2005). Higher altitude streams possess less diversity of the fish community, and fish biomass is less abundant (Ruiz-Olmo, 1998). Wider streams were mainly situated in riparian reserves located at low to moderate altitudes, where fishes are plentiful. Stream depth, again, showed a positive correlation. As mentioned earlier, deeper streams were mostly located in riparian reserves contain a higher congregation of prey items. Even though the number of trees and bank undergrowth cover were not significant, they are vital features of an otter’s habitat as they act as a buffer to disturbances and contribute to bankside diversity. Other models which described L. perspicillata presence equally across the landscapes included the proportion of bank with soil, altitude, stream edge, distance to human settlements, and bank tree GBH which has been explained.
The approach of preserving riparian reserves within oil palm plantation was a successful strategy in mitigating impacts of land-use changes for otters. Otters show resilience and possess the ability to adapt to modified habitats when they are present in oil palm dominated landscapes (Laws, 2016). One contributing factor to lower occupancy in forested areas was due the recent disturbance in heavily degraded forests, where the fragments were salvage-logged between 2013 and 2016 (Seaman et al., 2019). Therefore, otters may have prioritized riparian reserves as their habitat since the vegetation buffer had enough time to recover following previous disturbances during plantations establishment. This allows trees to mature and complexity of surrounding vegetation to be established, providing ample denning site, protection against predators, and prey stock to recover from disturbances (Laws, 2016).
Next is the availability of prey items in streams in oil palm dominated landscapes. This offers sustenance to otters as they travel across the landscape and was confirmed by numerous observations of spraints in riparian reserves. An extensive study on freshwater fishes discovered 28 species present in and around the SAFE Project area (Wilkinson, 2018), where up to 25 are known as focal food items to humans: primary forest (n=10), logged forest (n=13), heavily degraded forest (n=17), oil palm plantation with riparian reserves (n = 22), and oil palm plantation without riparian reserves (n=15). Focal fishes to local communities had an unexpected resilience to severe disturbance from land-use changes as the area was observed to have a relatively high level of species richness (Wilkinson, 2018; Wilkinson et al., 2018). Laws (2016) analyzed invertebrate abundance, where it was significantly lower in heavily degraded forests resulting in a limiting factor to otters there. There was a relationship between disturbance history and invertebrate abundance, implying that abundance might be negatively affected by the recent salvage-logging events. Since mature plantations had enough recovery time after the previous disturbances, it allows associated invertebrate communities to recuperate after the initial establishment of the plantation.
The influence of selective logging is still evident in stream environmental conditions even after 10 to 15 years following logging, when logged forests were compared to old-growth forests (Luke et al., 2017b). Streams in riparian buffers of oil palm plantations maintained more natural stream conditions compared to streams without buffers, displaying the value of preserving or restoring riparian buffers for the management of freshwater in these catchments. Although, it is not enough to fully protect streams from the impacts of oil palm agriculture. Another factor for otter resilience in agricultural site is because plantations without reserves can act as a corridor for otters to travel between reserves possessing better quality forests across the matrix (Bhagwat et al., 2008; Luskin and Potts, 2011). Mature plantations possess enough undergrowth cover to provide protection during movement. There is also more active roads between plantations and forests (continuous logged and heavily degraded), which might potentially cause an increased chance of conflicts between humans or transportation.
CONSERVATION IMPLICATIONS
The present study shows that otters have a high occupancy in agricultural sites. It suggests that oil palm plantations with riparian reserves have a potential as functional habitats for otters which are underestimated in the past. The establishment of sustainable oil palm plantation management might have greatly played a role in the preservation of otter habitats within an extremely modified landscape. The maintenance of riparian reserves in plantations given enough time to recuperate after past disturbances managed to mitigate the damaging impacts of agricultural intensification on freshwater habitats for otters.
Even though they can survive in these habitats, it should not replace the importance of intact forested habitats as they lack proper vegetation communities and are susceptible to extreme fluctuations in abiotic variables such as temperature and humidity, especially in streams without any vegetation buffer. The detection of signs across land-use types was highly influenced by the stream topography, bank substratum, and weather conditions. Therefore, supplementary sampling methods using advanced technologies, e.g. camera trap, eDNA analysis, radiotelemetry, are greatly needed to cover the limitations of traditional methods.
Since there is a limited suitable habitat for otters, especially within the monoculture landscape, this study highlights the importance of preserving riparian buffer strips adjoining water bodies. The two otter species detected during the study show a high resilience towards changes and managed to thrive well in agricultural sites and areas where humans are present as long as there are ample food and shelter. The evaluation of prey species stocks, especially in water bodies located adjacent to plantations, needs to be monitored appropriately. Wastes from both nearby palm oil mills and human settlements directly impact prey items. Therefore, it is important to observe the health of the riverine system as this will not only impact otters but also humans living within the area.
As otters are living nearby to humans, especially in plantations and urban areas, more cases of human-otter conflicts have been reported multiple times over the past years (Danau Girang Field Centre, 2020; Duplaix and Savage, 2018; Elankovan, 2019; Gomez and Bouhuys, 2018; Lokman, 2020; Lubis, 2005; Ottercity, 2020; Prakash et al., 2012; Prakash et al., 2014; Shenoy et al., 2003; Tan, 2015; Tan, 2020; Wai, 2019; Woo and Bakar, pers. comm., 2020). Therefore, these conflicts between both parties need to be addressed appropriately. Education and awareness programs amongst the local communities and plantation workers are a priority to educate on the importance of otters in the environment. State-wide social survey with fishermen and aquaculture pond owners should be conducted to identify the conflict intensity between human and otters within the fishery industry, and by creating a program to work alongside them in the future to reduce potential conflicts. The hunting intensity of otters is not known in Sabah, but it is essential to investigate how severe it is as Southeast Asia has the highest contribution towards illegal hunting and pet trades (Gomez et al., 2016; Gomez and Bouhuys, 2018). Negative perception towards otters should be reversed, and mitigation of human-otter conflict must be implemented throughout the state to aid in recovering the populations of Bornean otters.
Restoration and preservation of vegetation buffers strips, controlling pollution, and limiting extraction activities are vital in ensuring otter population survival in oil palm dominated landscapes. There is a need for enforcement for better monitoring of riparian buffer widths in plantations. Priority should be given to ensure oil palm plantation managements adhere to the regulations given that it is poorly applied at the moment. Retaining connectivity between reserves and intact forests through corridors should be an approach used in creating a wildlife-friendly plantation to reduce conflict between wildlife and humans during movement and to ensure genetic flow. The conversion of oil palm plantations from previously degraded landscapes should be a priority above intact forests, and that method to lessen the effects of conversion towards wildlife in logged forests must be established.
Acknowledgements: This study would not have been possible without the financial support from Universiti Malaysia Sabah under the UMSGreat postgraduate students' research grant scheme (GUG0075-STWN-2/2016). We are grateful to the SAFE Project management team and Institute for Tropical Biology and Conservation (ITBC), UMS for providing us with all the logistical support and field assistance throughout the data collection period. Malaysian Nature Society financially supported the authors (AP and AW) of this paper to attend the 14th International Otter Congress in China. Ms. Anna Laws and Dr. Matthew Struebig guided us during the initial stage of this research with fieldwork training. We also thanked Yayasan Sabah for permitting us to conduct the otter surveys at the SAFE Project area in Kalabakan (Project No. 202). Finally, we would like to extend our gratitude to the two anonymous reviewers for their valuable comments which we believe have improved the quality of this paper significantly.
REFERENCES
Aadrean, A., Usio, N. (2017). Small-clawed otters (Aonyx cinereus) in Indonesian rice fields: latrine site characteristics and visitation frequency. Ecological Research, 32: 899-908.
Anoop, K.R., Hussain, S.A. (2004). Factors affecting habitat selection by smooth-coated otters (Lutra perspicillata) in Kerala, India. Journal of Zoology London. 263: 417-423.
Anoop, K. R., Hussain, S. A. (2005). Food and feeding habits of smooth‐coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology, 266: 15-23.
Barton, K. (2020). Mu-MIn: Multi-model inference. R Package Version 1.43.17. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf.
Bedford, S.J. (2009). The effects of riparian habitat quality for European Otter (Lutra lutra) in Devon. Bioscience Horizons, 2(2): 125-133.
Bernard, H., Bili, R., Wearn, O. R., Hanya, G., Ahmad, A.H. (2014). The distribution and persistence of primate species in disturbed and converted forest landscapes in Sabah, Malaysia: Preliminary results. Annual Report of Pro Natura Fund, 22: 159-168.
Bernard, H., Bili, R., Matsuda, M., Hanya, G., Wearn, O.R., Wong, A., Ahmad, A.H. (2016). Species Richness and Distribution of Primates in Disturbed and Converted Forest Landscapes in Northern Borneo. Tropical Conservation Science, 9 (4).
Bhagwat, S.A., Willis, K.J., Birks, H.J., Whittaker, R.J. (2008). Agroforestry: a refuge for tropical biodiversity? Trends in Ecology and Evolution. 23: 261-267.
Burnham, K.P., Anderson, D.R. (2002). Model selection and multimodel inference:A practical information–theoretic approach (second ed.). New York: Springer-Verlag.
Cho, H.S., Choi, K.H., Lee, S.D., Park, Y.S. (2009). Characterising habitat preference of Eurasian river otter (Lutra lutra) in streams using a self-organising map. Limnology, 10: 203-213.
Danau Girang Field Centre (2020, September 9). Last February, we featured the first sighting of the hairy-nosed otter in the Kinabatangan (1st picture). Yesterday, our team found the body of a freshly-killed individual (2nd picture) along the Sandakan-Lahad Datu road near Batu Putih (Kinabatangan). The hairy-nosed otter is extremely rare and listed as Endangered in the IUCN Red List. The number of road kills will only increase with the current development of the Pan Borneo Highway. #roadkill #panborneohighway #hairynosedotter [Facebook update] Retrieved from https://www.facebook.com/permalink.php?story_fbid=3301838419883787&id=147476775319983.
Danielsen, F., Beukema, H., Burgess, N.D., Parish, F., Bruhl, C.A., Donald, P.F., Murdiyarso, D., Phalan, B., Reijnders, L., Struebig, M., Fitzherbert, E.B. (2009). Biofuel plantations on forested lands: double jeopardy for biodiversity and climate. Conservation Biology, 23 (2): 348-58.
Duplaix, N., Savage, M. (2018). The Global Otter Conservation Strategy. IUCN/SSC Otter Specialist Group, Salem, Oregon, USA. pp. 166.
Elankovan, V. (2019, March 4). Pictures of Two Otters Killed & Cruelly Hung by Fish Breeder in Penang Go Viral. [Social Stories] World of Buzz. Retrieved from https://worldofbuzz.com/pictures-of-an-otter-killed-cruelly-hung-by-fish-breeder-in-penang-go-viral/.
Environment Protection Department (EPD) (2011). Guidelines for minimising impacts of oil palm plantations and palm oil mills on quality of rivers in Sabah. Kota Kinabalu.
Ewers, R.M., Didham, R.K., Fahrig, L., Ferraz, G., Hector, A., Holt, R.D., Kapos, V., Reynolds, G., Sinun, W., Snaddon, J.L., Turner, E.C. (2011). A large-scale forest fragmentation experiment: The Stability of Altered Forest Ecosystems Project. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 3292–3302.
Feld, C.K., Birk, S., Bradley, D.C., Hering, D., Kail, J., Marzin, A., Melcher, A., Nemitz, D., Pedersen, M.L., Pletterbauer, F., Pont, D., Verdonschot, P.F.M., Friberg, N. (2011). In: Woodward, G. (Ed.), From Natural to Degraded Rivers and Back Again: a Test of Restoration Ecology Theory and Practice, first ed. Elsevier Ltd., Amsterdam, The Netherlands.
Fiske, I., Chandler, R. (2011). “unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance.” Journal of Statistical Software, 43(10), 1-23. http://www.jstatsoft.org/v43/i10/.
Foster, W., Snaddon, J., Turner, E., Fayle, T., Cockerill, T. D., Ellwood, F., Broad, G., Chung, A., Eggleton, P., Chey, V. K. & Yusah, K. M. (2011). Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 366: 3277-3291.
Foster-Turley, P. (1992). Conservation ecology of sympatric Asian otters Aonyx cineria and Lutra perspicillata. PhD Thesis. University of Florida. pp. 172.
Francis, C. (2008). A Guide to the Mammals of South-East Asia. Princeton University Press, Princeton, New Jersey, and Oxford, United Kingdom. 392 pp.
Gallant, D., Vasseur, L., Dumond, M., Tremblay, E.H., Bérubé, C. (2009). Habitat selection by river otters (Lontra canadensis) under contrasting land use regimes. Canadian Journal of Zoology, 87: 422-432.
Giam, X., Hadiaty, R.K., Tan, H.H., Parenti, L.R., Wowor, D., Sauri, S., Chong, K.Y., Yeo, D.C., Wilcove, D.S. (2015). Mitigating the impact of oil-palm monoculture on freshwater fishes in Southeast Asia. Conservation Biology, 29: 1357-1367.
Gomez, L., Leupen, B., Theng, M., Fernandez, K., Savage, M. (2016). Illegal Otter Trade: An Analysis of Seizures in Selected Asian Countries (1980-2015). TRAFFIC Southeast Asia.
Gomez, L., Bouhuys, J. (2018). Illegal Otter Trade in Southeast Asia. TRAFFIC Report. 1-36.
Gray, C.L., Slade, E.M., Mann, D.J., Lewis, O.T. (2014). Do riparian reserves support dung beetle biodiversity and ecosystem services in oil palm-dominated tropical landscapes? Ecol. Evol., 4: 1049–1060.
Gray, C.L., Lewis, O.T., Chung, A.Y.C., Fayle, T.M. (2015). Riparian reserves within oil palm plantations conserve logged forest leaf litter ant communities and maintain associated scavenging rates. Journal of Applied Ecology, 52: 31-40.
Hansen, M.C., Potapov, P.V., Moore, R., Hancher, M., Turubanova, S.A., Tyukavina, A., Thau, D., Stehman, S.V., Goetz, S.J., Loveland, T.R., Kommareddy, A., Egorov, A., Chini, L., Justice, C.O., Townshend, J.R.G. (2013). High-resolution global maps of 21st-century forest cover change. Science, 342: 850–53. Data available on-line from: http://earthenginepartners.appspot.com/science-2013-global-forest.
Hussain, S.A. (2016). Ecology of Asian Otters. Presentation at the 13th International Otter Congress, Singapore. IUCN SSC Otter Specialist Group. 3-8 July 2016. 35 pp.
Jo, Y.S., Won, C.M., Fritts, S.R., Wallace, M.C., Baccus, J.T. (2017). Distribution and habitat models of the Eurasian otter, Lutra lutra, in South Korea. Journal of Mammalogy, 98: 1105-1117.
Kamjing, A., Ngoprasert, D., Steinmetz, R., Chutipong, W., Savini, T., Gale, G.A. (2017). Determinants of smooth-coated otter occupancy in a rapidly urbanising coastal landscape in Southeast Asia. Mammalian Biology, 87: 168-175.
Khoo, M., Sivasothi, N. (2018). Population structure, distribution, and habitat use of smooth-coated otters Lutrogale perspicillata in Singapore. IUCN Otter Specialist Group Bulletin. 35: 171-182.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford: Oxford University Press. pp. 280.
Kruuk, H., Kanchanasaka, B., Wanghongsa, S., O’ Sullivan, S. (1994). Niche separation in three sympatric otters Lutra perspicillata, L. lutra and Aonyx cinerea in Huai Kha Khaeng, Thailand. Biological Conservation, 6 (1): 115-120.
Lamb, D., Erskine, P., Parrotta, J. (2006). Restoration of degraded tropical forest landscapes. Science. Science, 310: 1628-1632.
Laws, A. (2016). Investigating the occupancy of otter species in oil palm and forest estates in Sabah, Malaysia. Unpublished Master’s Thesis. Durrell Institute of Conservation and Ecology (DICE), School of Anthropology and Conservation, University of Kent, United Kingdom.
Lokman, M.I.N. (2020, June 3). Dapat dr group whatsap tapi nama kena sensor lah pulak Nasihatsy, nk buat kerja mcm nijgn tayang sgttak perlu bangga dgn benda2 mcm niwaloupun anda buat krn kesulitan yg di alami. Tak pasal² telah memburukkan nama penternak2 @ pemilik sangkar ikan yg lain. [Facebook update] Retrieved from https://www.facebook.com/story.php?story_fbid=3196455103733263&id=100001064154266.
Lubis, R. (2005). First recent record of hairy-nosed otter in Sumatra, Indonesia. IUCN Otter Specialist Group Bulletin, 22(1): 14-20.
Luke, S.H., Dow, R.A., Butler, S. Chey V.K., Aldridge, D.C., Foster, W.A., Turner, E.C. (2017a). The impacts of habitat disturbance on adult and larval dragonflies (Odonata) in rainforest streams in Sabah, Malaysian Borneo. Freshwater Biology. 62: 491-506.
Luke S.H., Barclay, H., Bidin, K., Chey, V.K., Ewers, R.M., Foster, W.A., Nainar, A., Pfeifer, M., Reynolds, G., Turner, E.C., Walsh, R.P.D., Aldridge, D.C. (2017b). The effects of catchment and riparian forest quality on stream environmental conditions across a tropical rainforestand oil palm landscape in Malaysian Borneo. Ecohydrology, 2017; e1827.
Luskin, M.S., Potts, M.D. (2011). Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic Appl. Ecol., 12, 540-551.
MacKenzie, D.I., Nichols, J.D., Boyle, J.A., Pollock, K.H., Bailey, L.L., Hines, J.E. (2006). Occupancy estimation and modelling: inferring patterns and dynamics of species occurrence. Academic Press.
Malaysian Metrological Department, Ministry of Science, Innovation, Technology, Environment and Climate Change, Malaysia (2019). Eilieen Cheah to Evyen Wevan Jebrail, May 10, 2019. Letter. Series JMM/CSBH/0/600-06/5 Jld.40 (81). pp. 7.
Mazerolle, M.J. (2020). AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-0, https://cran.r-project.org/web/packages/AICcmodavg/index.html.
Mendenhall, C.D., Daily, G.C., Ehrlich, P.R. (2012). Improving estimates of biodiversity loss. Biological Conservation, 151: 32-34.
Mitchell, S.L., Edwards, D.P., Bernard, H., Coomes, D., Jucker, T., Davies, Z.G., Struebig, M.J. (2018). Riparian reserves help protect forest bird communities in oil palm dominated landscapes. J. Appl. Ecol. 55: 2744-2755.
Ottercity (2020, September 6). [Death by fish hook]- Punggol pups just can’t get a break [Facebook update] Retrieved from https://www.facebook.com/ottercity/posts/876568066451421.
Ottino, P., Giller, P. (2004). Distribution, density, diet and habitat use of the otter in relation to land use in the Araglin Valley, Southern Ireland. Biology and Environment: Proceedings of the Royal Irish Academy. 104B: 1-17.
Peel, A. (2018). Avian stress across a gradient of forest degradation in Borneo. Unpublished master thesis, Imperial College London, England.
Pillai, A.S. (2015). Influence of different habitats on occurrence of asian small-clawed otter (Aonyx cinereal, Illeger, 1815) in Wayanad, Kerala, India. MSc Thesis. College of Veterinary and Animal Sciences, India.
Phillipps, Q., Phillipps, K. (2016). Phillipps’ Field Guide to the Mammals of Borneo and Their Ecology: Sabah, Sarawak, Brunei, and Kalimantan. Princeton University Press. Princeton and Oxford. 400pp.
Prakash, N., Mudappa, D., Shankar Raman, T.R., Kumar, A. (2012). Conservation of the Asian small-clawed otter (Aonyx cinereus) in human-modified landscapes, Western Ghats, India. Tropical Conservation Science. 5: 67-78.
Prakash, N., Perinchery, A., Nayak, R. (2014). Monitoring otter populations and combating poaching through stakeholder participation in India (Final Report). Nature Conservation Fund. pp. 1-31.
R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 29 December 2019.
Rasband, W.S. (2018). ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018.
Romanowski, J., Brzeziński, M., Zmihorski, M. (2012). Habitat correlates of the Eurasian otter Lutra lutra recolonizing Central Poland. Acta Theriol (Warsz), 58: 149-155.
Roundtable on Sustainable Palm Oil (RSPO). (2013). Adoption of principles and criteria for the production of sustainable palm oil.
Ruiz-Olmo, J. (1998). Influence of altitude on the distribution, abundance and ecology of the otter (Lutra lutra). In: Dunstone, N., Gorman, M. L. Behaviour and Ecology of Riparian Mammals (Eds.). Cambridge University Press, New York. pp. 159-176.
Sabah Water Resources Enactment (SWRE), (1998). LEX‐FAOC033659. http://www.fao.org/faolex/en/. Accessed on 21 May 2019.
Sabo, J.L., Sponseller, R., Dixon, M., Gade, K., Harms, T., Heffernan, J., Jani, A., Katz, G., Soykan, C., Watts, J., Welte. J. (2005). Riparian zones increase regional species richness by harboring different, not more, species. Ecology, 86: 56-62.
Seaman D.J.I., Bernard, H., Ancrenaz, M., Coomes, D., Swinfield, T., Milodowski, D.T., Humle, T., Struebig, M. J. (2019). Densities of Bornean orang‐utans (Pongo pygmaeus morio) in heavily degraded forest and oil palm plantations in Sabah, Borneo. American Journal of Primatology. 2019, e23030.
Sekercioglu, C.H., Loarie, S.R., Oviedo-Brenes, F., Mendenhall, C.D., Daily, G.C., Ehrlich, P.R. (2015). Tropical countryside riparian corridors provide critical habitat and connectivity for seed-dispersing forest birds in a fragmented landscape. Journal of Ornithology, 156: 343-353.
Shenoy, K., Varma, S., Prasad, K.V. (2003). Otters in Cauvery Wildlife Sanctuary, southern India: A study on the habitat selection and diet composition on the Smooth-coated otter (Lutra perspicillata). Nityata Foundation, and Asian Elephant Research and Conservation Centre (A Division of Asian Nature Conservation Foundation), C/o Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
Shenoy, K., Varma, S., Devi Prasad, K.V. (2006). Factors Determining Habitat Choice of the Smooth-coated otter, Lutra perspicillata in a South Indian River System. Current Science, 91(5): 637-643.
Struebig, M.J., Kingston, T., Zubaid, A., Mohd-Adnan, A., Rossiter, S.J. (2008). Conservation value of forest fragments to Palaeotropical bats. Biological Conservation, 141: 2112-2126.
Struebig, M.J., Turner, A., Giles, E., Lasmana, F., Tollington, S., Bernard, H., Bell, D. (2013). Quantifying the biodiversity value of repeatedly logged rainforest: Gradient and comparative approaches from Borneo. Advances in Ecological Research, 48: 183-224.
Tan, H.H. (2015). A roadkill record of a hairy-nosed otter (Lutra sumatrana) from Selangor, Peninsular Malaysia. IUCN Otter Specialist Group Bulletin. 32: 8-11.
Tan, M. Z. (2020, May 15). Singaporean actress Jazreel Low ‘heartbroken’ after wild otters feast on 13-year-old pet arowana (VIDEO). Malay Mail. Retrieved from https://www.malaymail.com/amp/news/showbiz/2020/05/15/singaporean-actress-jazreel-low-heartbroken-after-wild-otters-feast-on-13-y/1866381.
Theng, M., Sivasothi, N., Tan, H.H. (2016). Diet of the smooth-coated otter Lutrogale perspicillata (Geoffroy, 1826) at natural and modified sites in Singapore. The Raffles Bulletin of Zoology, 64: 290-301.
Thomas, J.W., Maser, C., Rodiek, J.E. (1979). Riparian zones. In: Thomas, J.W., Maser, C. (eds.) Wildlife habitats in managed rangelands the Great Basin of southeastern Oregon. Gen. Tech. Rep. PNW-80. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. pp. 18.
Turner, E.C., Snaddon, J.L., Fayle, T.M., Foster, W.A. (2008). Oil palm research in context: identifying the need for biodiversity assessment. PLoS ONE. 3(2):e1572.
Wai, L. (2019). Ecology of smooth-coated otter (Lutrogale perspicillata) in the Lower Kinabatangan Wildlife Sanctuary, Sabah, Malaysia. MSc. Thesis. Universiti Malaysia Sabah. Malaysia.
Wearn, O., Rowcliffe, M., Carbone, C., Pfeifer, M., Bernard, H., Ewers, R. (2017). Mammalian species abundance across a gradient of tropical land-use intensity: A hierarchical multi-species modelling approach. Biological Conservation, 212: 162-171.
Wilkinson, C.L. (2018). Protecting aquatic diversity in deforested tropical landscapes. PhD Thesis. Life Sciences, Imperial College London, United Kingdom. 213 p.p. Retrieved from https://spiral.imperial.ac.uk/handle/10044/1/65738.
Wilkinson, C.L., Yeo, D.C., Tan, H.H., Hadi Fikri, A., Ewers, R.M. (2018). The availability of freshwater fish resources is maintained across a land‐use gradient in Sabah, Borneo. Aquatic Conservation: Marine and Freshwater Ecosystems, 28(5): 1044-1054.
Wilkinson, C., Heon, S., Bignet, V., Fikri, A.H., Yeo, D., Tan, H.H., Ewers, R.M. (2020). All Fish catch data at the SAFE project 2011-2017. [Data set]. Zenodo.
Woo, C. Y, Bakar, F. (2020). Personal communication.
Résumé: Les Réserves Rivulaires servent de Refuge aux Loutres Asiatiques (Aonyx cinereus et Lutrogale perspicillata) dans les Paysages dominés par le Palmier à Huile au Sabah, sur l’Île de Bornéo en Malaisie
Nous avons déterminé l’occupation des espèces de loutres et évalué plusieurs caractéristiques de l’habitat influençant leur présence dans quatre types différents d’utilisation du sol : Forêts exploitées en Continu (FC), Forêts fortement Dégradées (FD), Réserves Riveraines dans des plantations de palmiers à huile (RR) et Plantations de palmiers à huile Sans réserves riveraines (PS). Notre objectif était de vérifier l’utilité de conserver une réserve rivulaire dans un paysage dominé par le palmier à huile pour la protection de la loutre. Cette étude a été menée dans l’État malaisien de Sabah, au nord de Bornéo. Nous avons étudié 36 sous-transects de cours d’eau dans tous les différents types d’utilisation du sol et avons détecté la présence de loutres en fonction de leurs trajectoires et de leurs pentes. Dans l’ensemble, deux des quatre espèces de loutres trouvées au Sabah ont été détectées dans les zones étudiées, à savoir la loutre cendrée, A. cinereus et la loutre à pelage lisse, L. perspicillata. Nous avons constaté que les cours d’eau des sites agricoles avaient une occupation de loutres significativement plus élevée que les zones boisées : RR (psi = 0,97), PS (0,83), FD (0,44) et FC (0,37). En utilisant la Modélisation Linéaire Généralisée (MLG), nous avons identifié que le territoire occupé par les loutres dans les paysages de palmiers à huile était positivement influencé par la disponibilité de grands arbres et toute autre végétation le long des berges. Les cours d’eau plus profonds étaient également davantage préférés par les loutres. Fait intéressant, une détection plus élevée d’indices de présence de loutre a été enregistrée le long des cours d’eau des plantations de palmiers à huile proches des habitations. D’une manière générale, cette étude suggère que les cours d’eau des plantations de palmiers à huile avec une végétation rivulaire sont un habitat favorable aux espèces de loutres. Par conséquent, le maintien des réserves rivulaires dans les plantations de palmiers à huile est une stratégie de gestion utile pour améliorer la conservation de la biodiversité dans un paysage agricole.
Revenez au dessus
Resumen: Las Reservas Ribereñas sirven como Refugio Crítico para las Nutrias Asiáticas (Aonyx cinereus y Lutrogale perspicillata) en Paisajes dominados por Palmera Aceitera Ee Sabah, Borneo Malayo
Determinamos la ocupación por las especies de nutria y evaluamos distintos rasgos del hábitat que influyen en su ocurrencia, en cuatro tipos de uso de la tierra: bosques explotados continuos (CF), bosques intensamente degradados (DF), reservas ribereñas dentro de plantaciones de palmera aceitera (RR) y plantaciones de palmera aceitera sin reservas ribereñas (OP). Nuestro objetivo fue evaluar la utilidad para la conservación de las nutrias, de retener una reserva ribereña en un paisaje dominado por palmera aceitera. Este estudio fue conducido en el estado Malayo de Sabah, porción norte de Borneo. Relevamos 36 sub-transectas en arroyos a lo largo y ancho de todos los tipos de uso de la tierra, y detectamos presencia de nutrias en base a huellas y fecas. En total, fueron detectadas en las áreas relevadas, dos de las cuatro especies de nutria que se encuentran en Sabah, concretamente la Nutria de Uñas Pequeñas Asiática, A. cinereus y la Nutria Lisa, L. perspicillata. Encontramos que los arroyos en sitios agrícolas tuvieron ocupación por nutrias significativamente más alta en comparación con las áreas forestadas: RR (psi = 0.97), OP (0.83), DF (0.44), y CF (0.37). Utilizando Modelado Lineal Generalizado (GLM) identificamos que la ocupación por nutrias en los paisajes de palmera aceitera estaba influenciada positivamente por la disponibilidad de grandes árboles y otra vegetación a lo largo de las barrancas. También fueron preferidos por las nutrias los arroyos más profundos. En forma interesante, en los arroyos en plantaciones de palmera aceitera ubicados más cerca de asentamientos humanos registramos más detección de signos de nutria. En general, este estudio sugiere que los arroyos en plantaciones de palmera aceitera con vegetación ribereña fueron útiles para las especies de nutria. Por lo tanto, retener reservas ribereñas dentro de plantaciones de palmera aceitera es una estrategia de manejo útil para mejorar la conservación de la biodiversidad en el paisaje agrícola.
Vuelva a la tapa
Rumusan: Rizab Riparian Berfungsi Sebagai Tempat Perlindungan Yang Kritikal Untuk Memerang Asia (Aonyx Cinereus Dan Lutrogale Perspicillata) Dalam Landskap Yang Telah Didominasi Oleh Kelapa Sawit Di Sabah, Borneo Malaysia
Kajian ini menentukan penghunian spesies memerang dan menilai beberapa ciri-ciri habitat yang menpengaruhi kehadiran mereka di dalam empat jenis penggunaan tanah yang berbeza: hutan berterusan yang telah dibalak (CF), hutan yang sangat terdegradasi (DF), rizab riparian dalam ladang kelapa sawit (RR), dan ladang kelapa sawit tanpa rizab riparian (OP). Tujuan kajian ini adalah untuk memastikan pentingnya rizab riparian dikekalkan di landskap yang telah didominasi oleh kelapa sawit untuk tujuan pemuliharaan memerang. Kajian ini dilakukan di negeri Sabah, Malaysia iaitu di bahagian utara Borneo. Sebanyak 36 sub-transek anak sungai telah ditinjau yang merentasi semua jenis penggunaan tanah yang berbeza dan kehadiran memerang dikesan berdasarkan bekas tapak kaki dan najis mereka. Secara keseluruhan, dua daripada empat spesies memerang yang terdapat di Sabah berada di dalam kawasan yang ditinjau, iaitu Memerang Kecil, A. cinereus dan Memerang Licin, L. perspicillata. Anak sungai di kawasan pertanian didapati memiliki penghunian memerang yang jauh lebih tinggi berbanding dengan kawasan hutan: RR (psi = 0.97), OP (0.83), DF (0.44), and CF (0.37). Dengan menggunakan Generalised Linear Modelling (GLM), kami mengenalpasti bahawa penghunian memerang di landskap kelapa sawit adalah dipengaruhi secara positif oleh kehadiran pokok-pokok besar dan vegetasi lain di sepanjang tebing. Anak sungai yang lebih dalam juga menjadi pilihan dan lebih disukai oleh memerang. Lebih menarik lagi, anak sungai di ladang kelapa sawit yang terletak berdekatan dengan penempatan manusia mencatatkan kehadiran tanda-tanda memerang yang lebih tinggi. Secara umum, kajian ini menunjukkan bahawa anak sungai di dalam ladang kelapa sawit yang mempunyai vegetasi riparian merupakan habitat yang berguna untuk spesies memerang. Oleh itu, dengan mengekalkan rizab riparian di dalam ladang kelapa sawit merupakan strategi pengurusan yang berguna dalam meningkatkan pemuliharaan biodiversiti di dalam landskap pertanian.
Kembali ke puncak