IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Citation: Awasthi, B., Banjade, B., Pandey, N., Joshi, S., Savage, M., Shrestha, P.M., Rawat, Y.B., and Bhatt, P.R. (2024). The Effects of Biological Water Quality on the Presence of the Smooth-Coated Otter in Far Western Nepal. IUCN Otter Spec. Group Bull. 41 (2): 71 - 87
The Effects of Biological Water Quality on the Presence of the Smooth-Coated Otter in Far Western Nepal
Balram Awasthi1, 2, 3, Baburam Banjade4, Nirmala Pandey5, Sarita Joshi5, Melissa Savage6, Purna Man Shrestha7, Yam Bahadur Rawat8, and Pusp Raj Bhatt5*
1Department of Zoology, Siddhanath Science Campus, Tribhuvan University, Mahendranagar, Nepal
2CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan 666303 China
3University of Chinese Academy of Sciences, Beijing 100049, China
4Nepal Zoological Society, Lumbini Province, Nepal
5Department of Microbiology, Siddhanath Science Campus, Tribhuvan University, Mahendranagar, Nepal
6Department of Geography, University of California, Los Angeles, USA
7Wildlife Research and Education Network, Kathmandu Nepal
8Department of National Park and Wildlife Conservation, PO Box:860, Babarmahal, Kathmandu, Nepal
*Corresponding Author Email: Pusprajbhatt2803@gmail.com
Received 27th June 2023, accepted 30th November 2023
Abstract: Otters are a key indicator species for assessing ecological integrity and are highly vulnerable to habitat alteration and environmental pollution. The Smooth-coated otter inhabits both terrestrial and aquatic habitats, preferring shallow water, soft sand and clay riverine banks, and riparian vegetation with good coverage. In our study, we conducted field surveys and analyzed various factors such as water quality, human disturbance, and vegetation structure to to investigate the correlation between otter presence and these parameters.This study reported that Smooth-coated otter habitat in the western Terai is influenced by water quality, vegetation structure, and human activities. Human disturbance has a negative relationship with otter presence, whereas tree canopy is positively correlated with otter presence. The water quality paramete rs (temperature ranging around 37-38 °C, pH around 8, Dissolved Oxygen ranging from 5.12 -5.91 mg/L, Biological Oxygen Demand > 3.35 to 4.55 mg/L and a high concentration of chloride and hardness are the preferred habitat conditions for the Smooth-coated otter. Microbe concentration in the water appears to have no relation with otter presence. This study suggests that riparian vegetation and water quality is likely to affect the capacity of a river or wetlands to support otter populations, and habitat restoration can encourage their return to areas where they are currently absent. Regular monitoring of water quality and vegetation, together with reduced anthropogenic pressures, are urgent needed to maintain long-term population and habitats of Smooth-coated otter in river basins of western lowlands of Nepal.
Keywords:Smooth-coated otter, water quality, human disturbance, vegetation, Far western Nepal
INTRODUCTION
Otters are a top predator and keystone species in freshwater ecosystems and considered to be important biological indicators of wetland health (Kruuk, 2006). The Smooth-coated otter (Lutrogale perspicillata) inhabits both terrestrial and aquatic habitats, preferring shallow water, soft sand and clay riverine banks, and riparian vegetation with good coverage (Acharya and Lamsal, 2010). The distribution of potential Smooth-coated otter habitat has been identified throughout the Terai region of Nepal, (Hodgson, 1839; Houghton, 1987; Acharya, 2006, Awasthi and Yoxon, 2018), but was predicted to be highly aggregated in protected areas, particularly in Bardia National Park, Suklaphanta National Park and Koshi Tappu Wildlife Reserve (Acharya and Lamsal, 2010; Acharya and Rajbhandari, 2014; Joshi et al., 2021; Acharya et al., 2023).
Smooth-coated otters are under threat from anthropogenic pressures such as habitat fragmentation and conflict with humans, including overfishing and habitat disturbance (Acharya and Rajbhandari, 2014). The Smooth-coated otter is listed as Vulnerable in the IUCN Red List (Acharya et al., 2023) and on Appendix I of the Convention on International Trade in Endangered Species.
Despite its importance as an indicator of aquatic habitat health (Foster-Turley et al., 1990; Yonzon, 1998), otter species in Nepal are inadequately addressed by conservation policies in comparison to mega-vertebrates (Acharya, 2017; Acharya et al., 2022). In the Terai Arc Landscape (TAL), the riverine indicator species are facing severe threats due to increasing human disturbances on water resources, such as irrigation intakes, bridges, sedimentation, over-fishing, habitat destruction, sand and stone mining, extraction of shoreline vegetation, firewood and grass cutting, livestock grazing, movement of people in Ghats, water pollution, and climate change (Acharya and Rajbhandari, 2014; Thapa et al., 2020; Joshi et al., 2021; Acharya et al., 2022; Shrestha et al., 2023).). Wetlands face various challenges, including reduction in area, sediment deposition, eutrophication, pollution, and contamination by harmful substances from untreated waste disposal (Niraula, 2012; Pant et al., 2019; Bhatta et al., 2022). These factors contribute to the decline of otter populations and a reduction in their distribution range (Acharya and Rajbhandari, 2014; Thapa et al., 2020). The presence of otters in freshwater ecosystems is linked to good water quality and vegetation (Kruuk, 2006). The deterioration of water quality directly impacts the food sources of otters, such as fish and crustaceans (Peterson and Schulte, 2016; Scorpio et al. 2016; de Almeida & Ramos Pereira, 2017).
When assessing otter populations and distribution, it is important to investigate water quality. This involves monitoring the physical and chemical factors such as pH, salinity, oxygen concentration, and turbidity, as well as biological parameters such as the invertebrate community and thermo-tolerant coliforms (Bartram and Balance, 1996; Bedford, 2009). Additionally, the quality of water in terms of physical, chemical, and biological aspects varies based on factors such as basin shape and size, depth, light penetration, precipitation, location, temperature, surrounding soil composition, dissolved minerals, and pH (Bedford, 2009; de Almeida and Ramos Pereira, 2017).
There is little information on such relationship of water quality with the spatiotemporal occurrence of Smooth-coated otters in Nepal. (Acharya and Rajbhandari, 2014). Otter populations are usually monitored by assessing their sprainting activity along rivers (Ottino and Giller, 2004; McCafferty, 2005; Preston et al., 2006). However, research shows that sprainting varies seasonally and thus may not give an accurate assessment of population distribution (Yoxon and Yoxon, 2014). Knowledge on habitat requirements, i.e. water quality, vegetation cover and stream flow, is crucial to the conservation and management of this threatened riverine indicator species. This study explores how water quality, vegetation cover and river flow influence the occurrence of Smooth-coated otters (based on sign survey and local knowledge on the population trends) in Terai region of western Nepal.
MATERIALS AND METHODS
Study Area
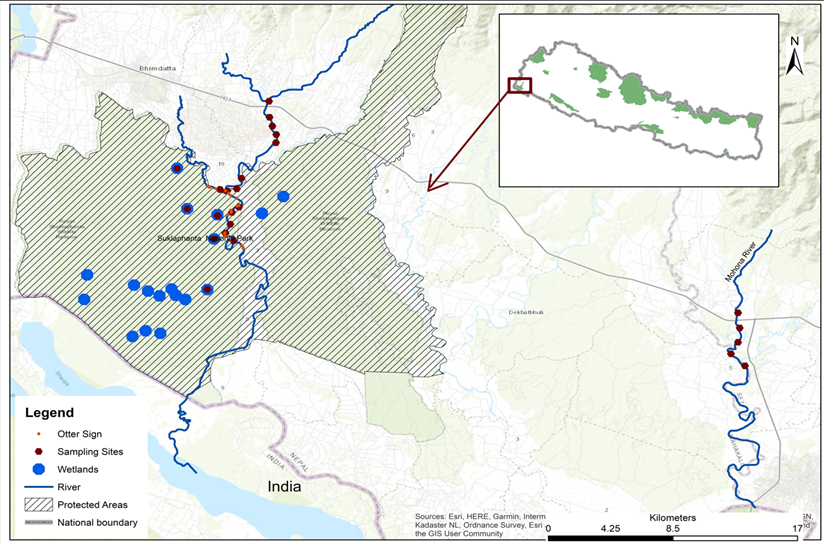
The study was carried out in Shuklaphanta National Park (ShNP) of Kanchanpur District and Mohana River of Kailali District, Sudurpaschim Province Nepal. The ShNP covers 305 km2 with open grasslands, riverbeds, and mixed forests and is surrounded by a buffer zone (243.5 km2) with similar characteristics (DNPWC, 2019). The study focused five site area with previous records of otters 1) Chaudhar River inside the ShNP (ShNP(R), 2) Chaudhar River junction, river entry area of the Park (ShNP(RJ), 3) lakes within the ShNP: Rani Tal, Salgaudi Tal, Baba Tal, and New Pokhari, hereafter collectively called wetlands (ShNP(W). The river stretches where otters were believed to be absent 4). Chaudhar River outside the ShNP settlement site (SR), 5) the Mohana River (MR), which originates in the Mahabharat Range and acts as a boundary between Kailali and Kanchanpur Districts (Fig. 1). The climate of the study areas is subtropical with an average maximum temperature of 37 °C and the average minimum of 7 °C. Annual rainfall ranges up to 2,016 mm.
Water Sampling and Analysis
Water samples were systematically collected at depths of 20-30 cm at locations in the study rivers and wetlands in March 2022. Altogether, 54 samples of water were collected; among these 39 samples were collected near recorded otter sign. Sterile polyethylene bottles with a capacity of 1 litre were used to collect water samples. Samples were acidified immediately by adding 2 ml of concentrated HNO3 as described by the American Public Health Association (APHA 2005). The samples were delivered to the laboratory of Siddhanath Science Campus, Tribhuvan University, Nepal. Until analysis, all samples were kept refrigerated at 4 °C. The samples were filtered using a 0.45 μm milli-pore nitrocellulose filter and the filtrate was collected in 20 mL HDPE bottles (Pant et al., 2019). pH, temperature, dissolved oxygen, biological oxygen demand, chloride, and hardness are important parameters affecting aquatic organisms and ultimately have direct or indirect effects on the water quality, aquatic diversity and, potentially, on otter presence. Therefore, these parameters were measured in the collected samples. Water temperature (WT) and pH were measured at sample sites using a digital meter. Chemical parameters, including hardness (H), dissolved oxygen (DO), biological oxygen demand (BOD), and chloride concentration were analyzed using standard methods (APHA, 2005). Microbiological analysis was performed using the standard membrane filtration technique for total coliform counts and thermotolerant coliform. Isolates were identified by biochemical testing (Pal et al., 2019).
Habitat Parameters
Habitat variables were measured at each of the water sampling sites. Habitat variables included average depth, water channel width, width of channel with sandy/rocky/muddy bed, water flow velocity, bank side condition (based on human disturbances parameters such as pollution, livestock grazing, illegal sand mining, fishing, clothes washing, bathing infrastructures, construction and invasive plant species) (Table 1). Also, 16 plots of 10×10 m2 each were sampled in ShNP and 16 plots along the otter absent sites. Tree species occurring within each plot was reported. Shrubs and herbs were recorded from 5×5 m2 and 1×1 m2 plots respectively within each of the large plots. Plant species were identified with the help of experts. The water channel width of wetlands was considered as the width of the wetland with water area. Width® considered as the width of river including the width with sandy/rocky bed.
| Table 1: Predictor variables. | ||||
| Variable | Description | Measure | Type of Variable | |
| Flow | Water flow velocity |
1 – Slow
2 – Fast 3 – Stagnant |
Categorical | |
| Slope | Riverbank slope |
1 – Flat
2 – Gentle slope 3 – Steep slope |
Categorical | |
| Bank side condition | Human Disturbance Index (HDI)* |
1 – High disturbance
2 – Moderate disturbance 3 – No disturbance |
Categorical | |
| Depth | Average depth | Centimeter (cm) | ||
| Width | Water channel width | Meter (m) | ||
| Width_shoreline | River width with sandy/rocky shoreline | Meter (m) | ||
| Otter | Presence | PR | ||
| Absence | AB | |||
| * Disturbance factor: 0 = no disturbance, 1-3 moderate disturbance and >3 high disturbance. | ||||
Data Analysis
This study employed descriptive analysis, including the calculation of mean, standard deviation, and standard error of the mean. Additionally, a water sampling location and otter distribution map was created using Arc Map 10.3. The statistical analysis was performed using SPSS version 20 software. One-way ANOVA was conducted for Dissolved Oxygen (DO), and the Kruskal-Wallace Test was conducted for Temperature, pH, BOD, and Chloride. However, there were insufficient samples for a hardness analysis. The association between otters and microbial parameters was examined using the Chi-Square Test. Canonical correspondence analysis (CCA) (Ter Braak 1987), an extension of reciprocal averaging and DCA, was used to relate otter species and water quality to the vegetation variables described in the vegetation analysis. With CCA, the species ordination axes are constrained to be linear combinations of specified environmental variables (Ter Braak, 1987). The association between the most important vegetation variables (identified by CCA) and otter species presence/absence at five sites was assessed using Spearman rank correlation coefficients. The relationship between otter presence and water quality and vegetation variables was visualized using a biplot of otter presence/absence and vegetation variables (Fielding and Brusven, 1993).
RESULTS
Physical Status of Wetlands and River
Physical parameters, average water depth (cm), water channel width (m), river width (m), water flow velocity, bank slope, and bankside condition and otter presence or absence are given in Table 2. The depth of wetland was 92.57 (cm) ± 2.11 followed by Chaudhar River, Mohana River and Chaudhar River (Settlement Site) (Table 2). Similarly, the water channel was wider in wetlands (average width of the wetland, Hruby, et al., 1999), followed by Chaudhar Junction, river and settlement sites, and the Mohana River. Riverbank slopes at all river sites were flat to gentle and the wetland sites were flat. The habitat condition was good in wetlands and the Chaudhar River of ShNP, but poor to moderate in human settlement sites of the Chaudhar River and Mohana River (Table 2).
Physico-Chemical Parameters of Water
There was significant variation in all physico-chemical parameters of water among different sites (P<0.001) (Table 3). Temperature was high in ShNP (RJ), followed by ShNP (R) and ShNP (W) and low in the MR (Table 1). Similarly, pH was found near 8 in all sites except MR, at 7.80.
DO value was found the highest in ShNP(W) followed by ShNP(RJ), ShNP(R), MR and lowest in SR, but there were no significant differences among ShNP(W), ShNP(R), and ShNP(RJ). Similarly, BOD was found highest in ShNP(R) followed by ShNP(RJ), ShNP(W), SR and lowest in MR. Chloride was found to be high at ShNP(R), followed by ShNP(RJ), ShNP(W), SR and lowest in MR. Water hardness was higher in ShNP(R), and lowest in ShNP(RJ) (Table 3).
Otter Presence correlated with Microbial Parameters
Eight microbes were analyzed to correlate with otter presence. Based on a chi square test, there was no relationship between otter presence or absence with microbial parameters. However, the Pearson chi square value was high in Shigella dysenteries followed by Bacillus subtilis, Escherichia faeclis, E. coli, K. pneumoniae, Salmonella paratyphi, Salmonella typhi and lowest for Vibrio cholera (Table 4).
Vegetation Composition and Environmental Factor (CCA)
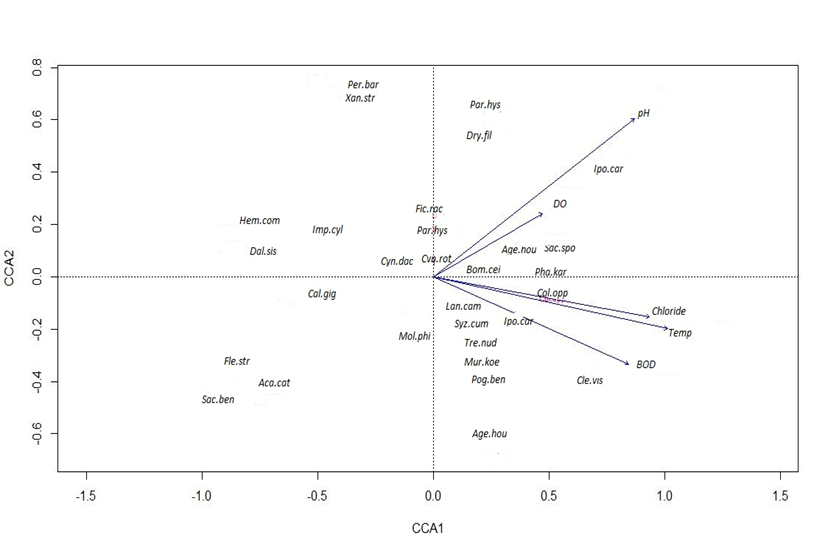
Bankside vegetation of wetlands and rivers may have impacts on water quality parameters. The CCA biplot shows that six species Parthenium hysterophorus, Dryopteris filix, Ipomea carnea, Ageratum houstonianum, Scchharum spontaneum, and Bombex ceiba were positively associated with high pH and DO with waters. The species Persicaria barbata, Xanthium strumarium, Imperata cylindrical, Cynodon dactylon, Hemarthria compressa, Dalbergia sisoo were negatively associated with pH and DO. Similarly, twelve species, Ageratum houstonianum, Colebrookea oppositifolia, Murraya koenigii, Clerodendrum viscosum, Syzygium cumini, Lantana camara, Ipomea carnea, Bombex ceiba, Trewia nudiflora, Flemingia strobilifera, Acacia catcheu, and Clerodendrum viscosum were positively associated with chloride, temperature, and BOD. The species Mallotus philippinensis, Saccharum bengalensis, Flemingia strobilifera, and Acacia catcheu were negatively associated with chloride, temperature, and BOD (Fig. 2).
Effect of Vegetation on Otter Presence
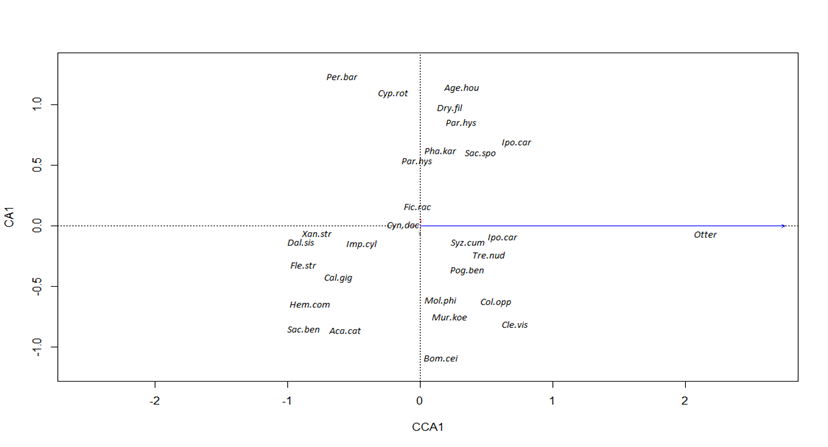
A CCA analysis was done to characterize the effect of vegetation on sign of otter presence. The CCA biplot in the Fig. 3 depicts otters present at sites with the species Ageratum houstonianum, Ipomoea carnea, Dryopteris filix, Parthenium hysterophorus, Sacchharum spontaneum, Ficus racemos, indicating a positive correlation. Also, Syzygium cumini, Pogostemon benghalensis, Mallotus philippinensis, Clerodendrum viscosum, Bombex ceiba, Colebrookea oppositifolia, and Murraya koenigii were also associated with otter presence.
Discussion
Habitat selection by Smooth-coated otters has been reported to be associated with water quantity (Scorpio et al., 2016) and complexity of riverbank vegetation (Pedroso et al., 2014), bankside canopy cover (Scorpio et al., 2016; de Almeida and Ramos Pereira, 2017), presence of mature vegetation for denning (McCafferty, 2005; Kruuk, 2006), and availability of prey and shelter for resting, grooming and breeding (Anoop and Hussain, 2004).
The Chaudhar River stretches outside the protected area (SR) and Mohana River were highly disturbed due to anthropogenic activities such as fishing and sand and gravel extraction, at a greater intensity of human disturbance as compared with habitat inside the protected area (Awasthi, unpublished data; Khan et al., 2014). In this study, otter sign was not found along the Chaudhar River located outside the protected area and Mohana River (Fig. 1). The presence of otters in the riverine ecosystem of Kailali districts was mentioned by Suwal and Verheught (1995). Local fishers have also indicated a potential presence of otters in the river, although they have not observed any signs recently. However, one fisherman reported observing an otter about 30 years ago. But in recent time, otters have not been reported as present in the Mohana River and no evidence of their presence has been found. We did not analyze the causes for absence of otter along the Mohana River, but the characteristics of water and vegetation were analyzed. In addition to the effects of vegetation and water quality, another reason for the absence of otters may be a limitation of food resources in the river (Bedford, 2009; de Almeida and Ramos Pereira, 2017). According to local people, there are only small fish in this part of the Mohana River. There are also high levels of human disturbance in the Mohana River, including fishing, grazing, sand extraction, bathing and clothes washing (Thapa et al., 2020; Joshi et al., 2021; Acharya et al., 2022). A detailed study of otter presence, using camera traps, is needed to confirm the presence or absence of otters in the Mohana River, considered a potential site for otter presence. Other rivers and wetlands in Kanchanpur and Kailali, located in the same landscape and watershed, have provided evidence of otter presence. Otter signs on the Chudhar River and wetlands of ShNP, where otters are documented in undisturbed portions of the protected area, indicate otter preference for undisturbed sites (Acharya and Lamsal, 2010).
The physical parameters of rivers and wetlands, such as average depth(cm), water channel width, slope, water velocity are similar between sites, with the exception of bank side condition, which are poor in the SR and MR. This appears to suggest that habitat regeneration could potentially reestablish otter populations where they currently appear to be absent (Bedford, 2009).
Overall, water quality parameter temperature, pH, DO, BOD, chloride and hardness did not differ significantly between otter presence and absence sites. However, the water quality parameters of temperature, pH, DO, BOD, chloride and hardness are higher in ShNP(W), ShNP(R), and ShNP(RJ) than in the Mohana River (MR) and Chaudhar human settlement sites (SR) (Table 3). Otters were present in sites with high values of these five water quality parameters. It appears that otters may prefer temperatures ranging near 37-38 °C, pH around 8, DO ranging from 5-6, BOD > 3 to 4 and high concentration of chloride and hardness (Table 3). The optimal ranges of water quality parameters support the growth and development of aquatic plants and animals (Clarke et al., 2003). Low levels of dissolved oxygen can lead to anoxic conditions, suffocating aerobic organisms (Bedford, 2009). Otters in coastal environments need fresh water for drinking and bathing (Abdul-Patah et al., 2014). High salinity concentrations are documented to have negative impacts on otters (Dias et al., 2022). A high concentration of organic matter can decrease dissolved oxygen availability, resulting in a decrease in the diversity and abundance of otter prey like fish and macroinvertebrates (Damanik-Ambarita et al., 2016; Papadaki et al., 2016). Monitoring water quality is crucial for managing riverine systems and promoting sustainable water use. This is essential for the conservation of freshwater ecosystems. Insufficient water quantity can adversely affect otters, particularly in relation to their food sources, such as fish abundance (Papadaki et al., 2016).
This study also examined the relationship between otter presence and vegetation cover. Contrary to previous studies (Thom et al., 1998; McCafferty, 2005; Preston et al., 2006), no significant relationship was found. Bankside areas with dense canopy cover, characterized by taller and older trees, offer more protection to riverbanks, resulting in less temperature variation and a greater diversity of food resources (Chase et al., 2016). These areas also supply nutrients and organic matter to watercourses (Swanson et al., 2017; Taniwaki et al., 2017) and are favorable for otter presence (Rheingantz et al., 2014).
The CCA biplot shows that six species Parthenium hysterophorus, Dryopteris filix, Ipomea carnea, Ageratum houstonianum, Scchharum spontaneum, and Bombex ceiba were positively associated with high pH and DO, indicating that leachates from these species contribute the water quality by increasing the pH and DO (Musser, et al., 2019).The species Persicaria barbata, Xanthium strumarium, Imperata cylindrical, Cynodon dactylon, Hemarthria compressa, Dalbergia sisoo were negatively associated with pH and DO. These species contribute chemicals through leaching that reduce the pH and DO (Saklaurs et al., 2022). Similarly, twelve species Ageratum houstonianum, Colebrookea oppositifolia, Murraya koenigii, Clerodendrum viscosum, Syzygium cumini, Lantana camara, Ipomea carnea, Bombex ceiba, Trewia nudiflora, Flemingia strobilifera, Acacia catcheu, and Clerodendrum viscosum were positively associated with chloride, temperature and BOD parameters. The species Mallotus philippinensis, Saccharum bengalensis, Flemingia strobilifera, Acacia catcheu were negatively associated with chloride, temperature and BOD. These species may release a lower amount of chloride and may shade the water to reduce temperature and reducing BOD.
The CCA biplot analysis indicates that otter sign was found near sites dominated by B. ceiba, M. philippensis, C. viscosum, C. oppositifolia, L. camara, S. cumini, I. carnea, S. spontaneum, A. houstonianum, and T. nudiflora. These tree species provide a suitable high canopy habitat for otters (Scorpio et al., 2016; de Almeida and Pereira, 2017). The CCA biplot (Fig. 2) also reveals the influence of vegetation on water quality and its association with otter habitat. Furthermore, there was no significant difference in plant species diversity between the sites. The study also examined the relationship between otter sign and microbial parameters in the water, but the chi-square test did not show any significant association (Table 4).
The sampled waterways along MR and SR were heavily used by humans; nonetheless, bankside vegetation is sufficient to provide cover for otters. If local otter populations were to migrate to these river stretches, and human disturbances reduced, they may provide suitable otter habitat.
Our study has several limitations that prevent a complete analysis of potential otter habitat. Other factors such as electrical conductivity, turbidity, total suspended solids, ammonia, nitrate, orthophosphate, and COD (Acharya and Rajbhandari, 2014) may be relevant parameters and have an impact on otter habitats, populations, and the overall ecosystem health of wetlands. In order to determine the suitability of the Mohana river for Smooth-coated otters, additional information and detailed surveys are needed. Future studies should focus on assessing the status, and potential habitat evaluation and restoration. Our knowledge of habitat restoration and restoration of otters and their ecological functions is limited, so it is important to further examine parameters of potential suitable habitat, particularly in terms of the impact of the species on other crucial ecological services.
Management Implications
Currently, there is insufficient knowledge regarding the restoration of degraded habitat and the prediction of rewilding otter species to sites where they have been extirpated. In this study, we examined water quality parameters and vegetation in sites with and without otter presence. Our findings suggest that the overall habitat quality of sites where otters were recently extirpated is potentially suitable for future recolonization or expansion Smooth-Coated otters. Otters are considered keystone species that significantly impact the structure and function of wetland ecosystems, enhanceing the ecological well-being of water resources. This study provides a valuable foundation for determining potential habitats of Smooth-coated otters in far western Nepal. The analysis of the habitat parameters presented here have management implications, by offering further characteristics of suitable otter habitats and supporting evidence for rewilding efforts in rivers and wetlands.
CONCLUSION
Our study indicates that otter habitat in the Western Terai region of Nepal is influenced by water parameters, vegetation structure, and human disturbances. Negative relationships were found between otter sign and fishing activities, livestock grazing, and sand and gravel extraction, while a positive correlation was observed between otter presence and tree canopy. Our analysis indicates that suitable habitat conditions of the water environment of the Smooth-coated otter include water temperatures ranging around 37-38°C, pH around 8, Dissolved Oxygen levels ranging from 5-6 mg/L, Biological Oxygen Demand between 3.35 to 4.55 mg/L, and high concentrations of chloride and hardness. Microbe concentration in the water was found to be unrelated to indications of otter presence. In sum, our study suggests that the presence of riverbank vegetation and water quality can impact the ability of rivers and wetlands to support otter populations, and that habitat regeneration may encourage otter to return to areas which they no longer inhabit. Urgent actions to conserve the Smooth-coated otter and its habitat in the western lowlands of Nepal include regular monitoring of water quality and vegetation, as well as managing anthropogenic activities. These research findings should be considered when developing otter reintroduction strategies and management plans in human-dominated landscapes.
Acknowledgements: This project was funded by University Grant Commission (SRDI-74/75-S&D-01), Nepal to Balram Awasthi. We would like to thank Shuklaphanta National Park for technically assisting in carrying out our field work, the Department of National Parks and Wildlife Conservation and Department of Forests and Soil Conservation for granting permission to carry out the research, and Siddhanath Science Campus for laboratory support. Special thanks go to Lal B. Thapa for his valuable input and suggestions. Also, Laxman Poudyal, Kavi Raj Bohara, Bharat Awasthi, Ganesh Rana,Anand Sunaha, Hema Joshi (Nepali Subject Facilitator), and other park personnel are acknowledged for their valuable support. We additionally thank the reviewers for their suggestions to improve the manuscript.
REFERENCES
Abdul-Patah, P., Nur-Syuhada, N., Md-Nor, S., Sasaki, H., Md-Zain, B. (2014). Habitat and food resources of otters (Mustelidae) in Peninsular Malaysia. AIP Conference Proceedings, 1614 (1):693–699. https://doi.org/10.1063/1.4895286
Acharya, P. (2006). Otter and wetland conservation in Nepal. In: Rimal, (eds.) Water Resources, Security and Sustainability. SEEP Water, Kathmandu, 144-149
Acharya, P.M., (2017). Status of smooth-coated otters (Lutrogale perspicillata) (Geoffroy, 1826) in the Khauraha River of Bardia National Park, Nepal. OTTER, The Journal of the International Otter Survival Fund,3, 23-37. https://www.academia.edu/43307115/International_Otter_Survival_Fund_Journal_Vol_3
Acharya, P.M., and Lamsal, P. (2010). A survey for smooth coated otter Lutrogale perspicillata on the river Narayani, Chitwan National Park, Nepal. Hystrix, the Italian Journal of Mammalogy, 21(2): 203 - 207. https://doi.org/10.4404/hystrix-21.2-4464
Acharya, P.M., Rajbhandari, S. (2010). Distribution and conservation status of otters in Nepal. Zoo Journal,2: 27–37. http://ruffordorg.s3.amazonaws.com/media/project_reports/Distribution%20and%20Conservation%20Status%20of%20Otters%20in%20Nepal.pdf
Acharya, P.M., Rajbhandari, S.L. (2014). Habitats of Lutrogale perspicillata in the Narayani River, Chitwan National Park, Nepal: Assessment of water quality. Journal of Indian Research, 2: 67–76. https://jir.mewaruniversity.org/wp-content/uploads/2021/03/Vol_2_issue_1_Jan_Mar_2014/7.pdf
Acharya, P.M., Saeung, S., Techato, K., Rimal, N., Gyawali, S., Neupane, D. (2022). Review of environmental policies and otter conservation in Nepal. IUCN Otter Spec. Group Bull.39 (1): 44-55. https://www.iucnosgbull.org/Volume39/Acharya_et_al_2022.html
Acharya, P.M., Tainiramit, P., Techato, K., Baral, S., Rimal, N., Savage, M., Campos-Arceiz, A., Neupane, D. (2023). Predicting the distribution and habitat suitability of the Smooth-coated Otter (Lutrogale perspicillata) in Lowland Nepal. Global Ecology and Conservation,46 e02578. https://doi.org/10.1016/j.gecco.2023.e02578
Anoop, K.R. and Hussain, S.A. (2004). Factors affecting habitat selection by smooth-coated otters (Lutra perspicillata) in Kerala, India. Journal of Zoology263: 417-423. https://doi.org/10.1017/S0952836904005461
APHA. (2005). Standard Methods for the Examination of Water and Wastewater. 21st ed. American Public Health Association, Washington D.C. ISBN-13: 978-0875530475
Awasthi, B., Yoxon, G.M. (2018). Current Status and Conservation Threat of Otter in Nepal - A Review. OTTER, the Journal of the International Otter Survival Fund, 4: 38-44. https://www.researchgate.net/publication/330845870
Bartram, J. and Ballance, R. (1996). Water Quality Monitoring: A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes. CRC Press: London, UK. https://www.who.int/publications/i/item/0419217304
Bedford, S.J. (2009). The effects of riparian habitat quality and biological water quality on the European otter (Lutra lutra) in Devon. Bioscience Horizons, 2: 125-133. https://doi.org/10.1093/biohorizons/hzp015
Bhatta, R., Gurung, S., Joshi, R., Tuladhar, S., Regmi, D., Kafle, B.K., Dahal, B.M., Kafle, K.R., Kayastha, R., Prasad, A., Tripathee, L., Paudyal, R., , Guo, J., , Kang, S., and Sharma, C.M. (2022). Spatio-temporal hydrochemistry of two selected Ramsar sites (Rara and Ghodaghodi) of west Nepal. Heliyon, 8(11): e11243. https://doi.org/10.1016/j.heliyon.2022.e11243
Chase, J.W., Benoy, G.A., Hann, S.W.R., Culp, J.M. (2016). Small differences in riparian vegetation significantly reduce land use impacts on stream flow and water quality in small agricultural watersheds. Journal of Soil and Water Conservation, 71 (3): 194–205. https://doi.org/10.2489/jswc.71.3.194
Clarke, R.T., Wright, J.F., Furse, M.T. (2003). RIVPACS models for predicting the expected macroinvertebrate fauna and assessing the ecological quality of rivers. Ecological Modelling, 160: 219 –233. https://doi.org/10.1016/S0304-3800(02)00255-7
Damanik-Ambarita, M.N., Lock, K., Boets, P., Everaert, G., Nguyen, T.H.., Forio, M.A.E., Musonge, P.L.S., Suhreva, N., Bennetsen, E., Landuyt, D., Dominguez-Granda, L., Goethals, P.L.M. (2016). Ecological water quality analysis of the Guayas River Basin (Ecuador) based on macroinvertebrates indices. Limnologica Ecology and Management of Island Waters,57: 27–59. https://doi.org/10.1016/j.limno.2016.01.001
de Almeida, L.R. and Ramos Pereira, M.J. (2017). Influence of the water quality on the occurrence of the Neotropical otter (Lontra longicaudis) (Olfers, 1818) in a human-altered river basin. Marine and Freshwater Research, 69(1). https://doi.org/10.1071/MF17020
Dias, S.J, White, P.J.C., Borker, A.S., Fernandes, N.V. (2022). Habitat selection of smooth-coated otters (Lutrogale perspicillata) in the peri-coastal, urbanised landscape of Goa, India. Mammal Research, 67(3):299–309. https://doi.org/10.1007/s13364-022-00639-1
DNPWC. (2019). Shuklaphanta National Park Newsletter. Department of National Park and Wildlife Conservation, Ministry of Forests and Soil Conservation.
Fielding, D. J., and Brusven, M. A. (1993). Grasshopper (Orthoptera: Acrididae) community composition and ecological disturbance on southern Idaho rangeland. Environmental Entomology, 22(1):71-81 https://doi.org/10.1093/ee/22.1.71
Foster-Turley, P., Macdonald, S., Mason, C. (1990). Otters: An Action Plan for their Conservation. IUCN, Gland, Switzerland, 126 pp. https://portals.iucn.org/library/node/6046
Hodgson, B.H. (1839). Summary description of four new species of otter. Journal of the Asiatic Society of Bengal, 8: 319–320. https://doi.org/10.1080/00222934009496750
Houghton, S.J. (1987). The smooth-coated otter in Nepal. IUCN Otter Specialist Group Bulletin, 2: 5–8. https://www.iucnosgbull.org/Volume2/Houghton_1987.html
Hruby, T., Granger, T., Teachout, E. (1999). Methods for Assessing Wetland Functions. Volume I: Riverine and Depressional Wetlands in the Lowlands of Western Washington. Part 2: Procedures for Collecting Data. Washington State Department Ecology Publication #99-116, Olympia, Washington. https://apps.ecology.wa.gov/publications/documents/99116.pdf
Joshi, G.K., R. Joshi, B. Poudel. (2021). Distribution and threats to Smooth-coated Otters Lutrogale perspicillata (Mammalia: Carnivora: Mustelidae) in Shuklaphanta National Park, Nepal. Journal of Threatened Taxa, 13 (11): 19475–19483. https://doi.org/10.11609/jott.7322.13.11.19475-19483
Khan, M.S., Dimri, N.K., Nawab A., Ilyas, O., Gautam, P. (2014). Habitat use pattern and conservation status of smooth-coated otters Lutrogale perspicillata in the Upper Ganges Basin, India. Animal Biodiversity and Conservation, 37: 69–76. https://www.researchgate.net/publication/263507124
Kruuk, H. (2006). Otters: Ecology, Behavior and Conservation, Oxford University Press: New York, USA. https://doi.org/10.1093/acprof:oso/9780198565871.001.0001
McCafferty, D.J. (2005). Ecology and conservation of otters (Lutra lutra) in Loch Lomond and The Trossachs National Park. Glasgow Naturalist,24 (3): 29–35. https://www.glasgownaturalhistory.org.uk/ll_papers/llproc_dmc.pdf
Musser, S.R., Grafe, J., Ortega-Achury, S.L., Ramirez-Avila, J.J. (2019). Influence of Riparian Vegetation on Stream Health and Water Quality. In Proceedings of the World Environmental and Water Resources Congress 2019, Pittsburgh, PA, USA, 19–23 May 2019; American Society of Civil Engineers: Reston, VA, USA. 21, 59–67. https://ascelibrary.org/doi/10.1061/9780784482346.006
Niraula, R. (2012). Evaluation of the Limnological Status of Beeshazar Lake, a Ramsar Site in Central Nepal. Journal of Water Resource and Protection, 4 (5): 256–263. http://dx.doi.org/10.4236/jwarp.2012.45028
Ottino, P., Giller, P. (2004). Distribution, density, diet and habitat use of the otter in relation to land use in the Aragalin Valley, Southern Ireland. Biology & Environment Proceedings of the Royal Irish Academy104B (1): 1 –17. https://doi.org/10.1353/bae.2004.a809873
Pal, K.B., Pant, R.R., Rimal, B., Mishra, A. D. (2019). Comparative assessment of water quality in the Bagmati River Basin, Nepal. Zoo Journal, 5: 68–78. https://doi.org/10.3126/zooj.v5i0.34919
Pant, R.R, Dhakal, M., Baral, U., Dangol, A., Dhakal, T.M., Thapa, L.B., Baral, U., Dangol, A., Chalaune, T.B., Pal, K.B. (2019). Water quality assessment of the Betkot Lake, Sudurpaschim Province, Nepal. North American Academic Research, 2 (12): 36-62. https://doi.org/10.5281/zenodo.3566682
Papadaki, K., Chatzinikolaou, Y., Lazaridou, M., Ioannou, A., Artemiadou, V. (2016). Water quality evaluation of the middle and lower reaches of Pinios River based on benthic macroinvertebrates and physicochemical parameters during the low flow and high flow season. Pp. 897–901 In: 8th Panhellenic Symposium of Oceanography & Fisheries, 4–8 June, Thessaloniki, Greece. (HCMR: Athens, Greece).
Pedroso, N.M., Marques, T.A., Santos-Reis, M. (2014). The response of otters to environmental changes imposed by the construction of large dams. Aquatic Conservation, 24 (1), 66–80. https://doi.org/10.1002/aqc.2379
Peterson, E.K., Schulte, B.A. (2016). Impacts of pollutants on beavers and otters with implications for ecosystem ramifications. Journal of Contemporary Water Research and Education, 157 (1), 33–45. https://doi.org/10.1111/j.1936-704X.2016.03212.x
Preston, S.J., Portig A.A., Montgomery, W.I., McDonald R.A., Fairley, J.S. (2006). Status and diet of the otter Lutra lutra in Northern Ireland. Biology & Environment Proceedings of the Royal Irish Academy, 106(1): 57-63. http://dx.doi.org/10.3318/BIOE.2006.106.1.56
Rheingantz, M.L., de Menezes, J.F.S., de Thoisy, B. (2014). Defining Neotropical otter Lontra longicaudis distribution, conservation priorities and ecological frontiers. Tropical Conservation Science,7 (2): 214–229. https://doi.org/10.1177/194008291400700204
Saklaurs, M., Dubra, S., Liepa, L., Jansone, D., Jansons, A. (2022). Vegetation Affecting Water Quality in Small Streams: Case Study in Hemiboreal Forests, Latvia. Plants (Basel). 11(10):1316. https://doi.org/10.3390/plants11101316
Scorpio, V., Loy, A., Di Febbraro, M., Rizzo, A., Aucelli, P. (2016). Hydromorphology meets mammal ecology: River morphological quality, recent channel adjustments and otter resilience. River Research and Applications, 32 (3): 267–279. https://doi.org/10.1002/rra.2848
Shrestha, P.M., Gwachha, S., Shrestha, S., Hamal, A., Tamang, T.T., Awasthi, B., Ghimire, S., Koju, R. (2023). People’s Perceptions of the Eurasian otter (Lutra lutra) Conservation in Mugu District Nepal. OTTER, Journal of the International Otter Survival Fund, 9(1): 128-143 https://www.researchgate.net/publication/373167668
Suwal, R.N. and Verheught, W.J.M. (1995). Enumeration of the mammals of Nepal. Biodiversity profiles project technical publication, 6. Euroconsult, Arnhem, Netherlands. ISBN: 9789073287075
Swanson, S., Kozlowski, D., Hall, R., Heggem, D., Lin, J. (2017). Riparian proper functioning condition assessment to improve watershed management for water quality. Journal of Soil and Water Conservation, 72 (2), 168–182. https://doi.org/10.2489/jswc.72.2.168
Taniwaki, R. H., Cassiano, C. C., Filoso, S., de Barros Ferraz, S. F., de Camargo, P. B., Martinelli, L. A. (2017). Impacts of converting low intensity pastureland to high-intensity bioenergy cropland on the water quality of tropical streams in Brazil. The Science of the Total Environment, 584–585: 339–347. https://doi.org/10.1016/j.scitotenv.2016.12.150
Ter Braak, C.F. (1987). The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio, 69: 69-77. https://doi.org/10.1007/BF00038688
Thapa, P., Bijaya, G.C.D., Bhandari, J., Devkota, B.P., Silwal, T., Can, L. (2020). Distribution, threats and community perception of otter in Shuklaphanta National Park, Nepal. OTTER, Journal of the International Otter Survival Fund, 7: 128-142. https://www.researchgate.net/publication/354604065
Thom, T. J., Thomas, C. J., Dunstone, N., & Evans, P. R. (1998). The relationship between riverbank habitat and prey availability and the distribution of otter (Lutra lutra) signs: an analysis using a geographical information system. In N. Dunstone & M. L. Gorman (Eds.), Behaviour and Ecology of Riparian Mammals (pp. 135–158). chapter, Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511721830.010
Yonzon, P.B. (1998). Baseline information on wildlife of the West Seti River Valley with emphasis on birds and mammals. A report submitted to West Seti Hydroelectric Project, SMEC Ltd. 17 pp
Yoxon, P., Yoxon, K. (2014). Estimating otter numbers using spraints: is it possible? Journal of Marine Biology., 1-6. https://doi.org/10.1155/2014/430683
ANNEX 1: CCA STATISTICS - VEGETATION COMPOSITION AND ENVIRONMENTAL FACTORS.>
Effect of Vegetation on Water Parameters
| Inertia | Proportion | p-Value | |
| Constrained | 0.4525 | 0.2186 | 0.001 |
| Unconstrained | 1.6172 | 0.7814 | |
Importance of Components
| CCA1 | CCA2 | CCA3 | CCA4 | |
| Eigenvalue | 0.2166 | 0.09869 | 0.06464 | 0.04174 |
| Proportion explained | 0.1046 | 0.04768 | 0.03123 | 0.02017 |
Biplot Scores for Constraining Variables
| CCA1 | CCA2 | CCA3 | CCA4 | |
| Temperature | 0.9423 | -0.1856 | -0.05138 | -0.23263 |
| pH | 0.8086 | 0.5643 | -0.107 | -0.01057 |
| DO | 0.4378 | 0.2262 | -0.29725 | -0.64163 |
| BOD | 0.7857 | -0.3109 | -0.38548 | -0.3704 |
| Chloride | 0.8703 | -0.1437 | -0.20325 | 0.1524 |
Effect of Water Parameters on Otter Presence
| Inertia | Proportion | |
| Constrained | 0.2083 | 0.1006 |
| Unconstrained | 1.8614 | 0.8994 |
Importance of Components
| CCA1 | CCA2 | CCA3 | CCA4 | |
| Eigenvalue | 0.2083 | 0.3692 | 0.2241 | 0.1634 |
| Proportion | 0.1006 | 0.1784 | 0.1083 | 0.07895 |
ANNEX 2: EFFECT OF VEGETATION ON OTTER PRESENCE
| Inertia | Proportion | P-value | |
| Constrained | 0.2333 | 0.1111 | 0.001 |
| Unconstrained | 1.8659 | 0.8889 |
Eigenvalues, and their Contribution to the Scaled Chi-Square
| CA1 | CA2 | CA3 | CA4 | CA5 | |
| Eigenvalue | 0.2333 | 0.3751 | 0.2118 | 0.1784 | 0.15251 |
| Proportion | 0.1111 | 0.1787 | 0.1009 | 0.0850 | 0.07265 |
Résumé: Les Effets de la Qualité Biologique de l’Eau sur la Présence De La Loutre à Pelage Lisse dans l’Extrême Ouest du Népal
Les loutres sont une espèce indicatrice clé pour évaluer l’intégrité écologique et sont très vulnérables à l’altération de l’habitat et à la pollution de l’environnement. La Loutre à pelage lisse habite à la fois des habitats terrestres et aquatiques, préférant les eaux peu profondes, des berges fluviales de sable mou et d’argile, ainsi qu’une végétation riveraine avec une couverture satisfaisante. Dans notre étude, nous avons mené des enquêtes sur le terrain et analysé divers facteurs tels que la qualité de l’eau, les perturbations humaines et la structure de la végétation afin d’étudier la corrélation entre la présence des loutres et ces paramètres. Cette étude a révélé que l’habitat de la loutre à pelage lisse dans l’ouest du Teraï est influencé par la qualité de l’eau, la structure de la végétation et les activités humaines. Les perturbations humaines ont une corrélation négative avec la présence des loutres, alors que le couvert forestier est positivement corrélé à la présence des loutres. Les paramètres de la qualité de l’eau rs (température autour de 37-38 °C, pH autour de 8, oxygène dissous allant de 5,12 à 5,91 mg/L, demande biologique en oxygène > 3,35 à 4,55 mg/L, une concentration en chlorure et une dureté élevée) sont les conditions d’habitat préférées pour la loutre à pelage lisse. La concentration de bactéries dans l’eau ne semble avoir aucun rapport avec la présence de la loutre. Cette étude suggère que la végétation riveraine et la qualité de l’eau sont susceptibles d’affecter la capacité d'une rivière ou d’un milieu humide à accueillir les populations de loutres, et la restauration de l’habitat peut encourager leur retour dans des zones où elles sont actuellement absentes. Une surveillance régulière de la qualité de l’eau et de la végétation, ainsi qu’une réduction des pressions anthropiques, sont, de toute urgence, nécessaires pour maintenir à long terme la population et les habitats de la loutre à pelage lisse dans les bassins fluviaux des basses terres de l’ouest du Népal.
Revenez au dessus
Resumen: Efectos de la Calidad Biológica del Agua en la Presencia de la Nutria Lisa en el Extremo Oeste de Nepal
Las nutrias son especies indicadoras clave para evaluar la integridad ecológica y son altamente vulnerables a la alteración del hábitat y la contaminación ambiental. La nutria lisa vive en hábitats tanto terrestres como acuáticos, prefiriendo aguas someras, barrancas ribereñas blandas de arena y arcilla, y vegetación riparia con buena cobertura. En nuestro estudio, condujimos relevamientos de terreno y analizamos varios factores como la calidad del agua, el disturbio humano y la estructura de la vegetación, para investigar la correlación entre la presencia de nutrias y éstos parámetros. Este estudio concluyó que el hábitat de la Nutria lisa en Terai occidental está influenciado por la calidad del agua, la estructura de la vegetación, y las actividades humanas. El disturbio humano tiene una relación negativa con la presencia de nutrias, mientras que las copas de los árboles (el canopeo) se correlacionan positivamente con la presencia de nutrias. Los parámetros de calidad del agua (temperatura alrededor de 37-38 °C, pH alrededor de 8, Oxígeno Disuelto entre 5.12-5.91 mg/L, Demanda Biológica de Oxígeno > 3.35 a 4.55 mg/L y una alta concentración de cloro y la dureza) son las condiciones de hábitat preferidas por la nutria Lisa. La concentración microbiana en el agua parece no tener relación con la presencia de nutrias. Este estudio sugiere que es probable que la vegetación riparia y la calidad del agua afecten la capacidad de un río o de un humedal para sostener poblaciones de nutrias, y la restauración de hábitats puede favorecer su retorno a áreas en las cuales están actualmente ausentes. El monitoreo regular de la calidad del agua y la vegetación, junto con la reducción de las presiones antropogénicas, son necesarios en forma urgente para mantener a largo plazo a la población y el hábitat de la Nutria Lisa en las cuencas hídricas de las tierras bajas occidentales de Nepal.
Vuelva a la tapa
लेखसार
ओतहरू पारिस्थितिक प्रणालीको गुणस्तर र अखण्डता मूल्याङ्कन गर्नका लागि महत्त्वपूर्ण सूचक प्रजातिहरू हुन् तर हाल वासस्थानको विनाश र वातावरणीय प्रदूषणको कारण अत्यधिक जोखिममा छन् । खैरो ओत (स्मूथकोटेड ओटर) दुबै स्थलीय र जलीय वासस्थानहरूमा बस्छन् तर मुख्यतः पानी, बालुवा र माटो, नदीको किनारहरू र घना वनस्पतिहरु भएको स्थानलाई प्राथमिकता दिन्छन् । यस अध्ययनमा हामीले ओत को उपस्थिति र अन्य कारक तत्व को बिचमा कस्तो सम्बन्ध छ भनी जान्नको लागि, क्षेत्र अध्ययन, पानीको गुणस्तर , मानवीय क्रियाकलाप र वनस्पतिको उपस्थिति जस्ता विभिन्न कारक तत्वहरु को विश्लेषण गर्यौ । यस अध्ययनले पश्चिमी तराइमा खैरो ओत को वासस्थान, नदी,तालको पानीको गुणस्तर, वनस्पति संरचना र मानव गतिविधिबाट प्रभावित भएको देखाएको छ । मान्छेको उपस्थितीसँग ओतको उपस्थिति नकारात्मक सम्बन्ध छ, जबकि घना रूखहरुको उपस्थिति सकारात्मक रूपमा ओत उपस्थितिसँग सम्बन्धित छ । पानीको गुणस्तर मापदण्डहरू ( लगभग ३७-३८ डिग्री सेल्सियसको तापक्रम, pH-८ वरिपरि, घुलनशील अक्सिजन ५.१२-५.९१ mg/L, जैविक अक्सिजनको माग > ३.३५ देखि ४.५५ mg/L र उच्च क्लोराइड र कडापना भएको पानी ओतको वासस्थान को लागि उपयुक्त रहेको देखिन्छ । खैरो ओत को लागी वासस्थानमा पानीमा पाउने सूक्ष्म जीवहरूको घनत्वले ओतको उपस्थितिसँग कुनै सम्बन्ध छैन । यस अध्ययनले नदी वा सिमसार क्षेत्रको पानीको गुणस्तर र किनारामा पाउने वनस्पतिको संरचनाले ओतको उपस्थितिलाई असर गरेको देखिन्छ र वासस्थान पुनर्स्थापनाले उनीहरूलाई हाल अनुपस्थित रहेका क्षेत्रमा फर्कन प्रोत्साहित गर्न सक्छ । पश्चिम तराईको तल्लो भूभागका नदी बेसिनहरूमा खैरो ओत को वासस्थान र दीर्घकालीक जनसंख्यालाई जोगाउन तत्काल पानीको गुणस्तर र वनस्पतिको नियमित अनुगमनका साथै नदी , सिमसार क्षेत्रमा मानवीय क्रियाकलाप कमी गर्न आवश्यक छ ।
सुरुमा फर्कनुहोस्







