IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Citation: Somers, M.J., Loggenberg, J.L., and McIntyre, T. (2024). First Record of Gastrointestinal Parasites of African Otters. IUCN Otter Spec. Group Bull. 41 (2): 88 - 96
First Record of Gastrointestinal Parasites of African Otters
Michael J. Somers1*, Jancke L. Loggenberg2, and Trevor McIntyre2,3
1Eugène Marais Chair of Wildlife Management, Mammal Research Institute, Centre for Invasion Biology, Department of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
2Mammal Research Institute, Department of Zoology and Entomology, University of Pretoria, Pretoria,
South Africa
3Department of Life and Consumer Sciences, University of South Africa, Roodepoort, South Africa
*Corresponding Author Email: michael.somers@up.ac.za
Received 23rd Ocotber 2023, accepted 6th December 2023
Abstract: Parasites are found in many mammalian species, particularly in the gastrointestinal tract. They can spread from one species to another and cause severe diseases in some species. Parasite spread is especially important between wild and domestic animals because it can affect human health. In this study, the first record of gastrointestinal parasites in African otters is reported from South Africa. The two otter species investigated were the African clawless otter (Aonyx capensis) and the spotted-necked otter (Hydrictis maculicollis). We identified the parasite species in collected faeces and examined the differences in parasitic loads between the species and two distinct habitats. Scats were collected from latrine sites in two areas (a rural nature reserve and an urban park) and examined using Teleman’s concentration-sedimentation formalin/ether method. Parasites were identified up to the species level where possible, and loads were calculated. As we only found African clawless otter faeces in the natural area and spotted-necked otter faeces in the urban reserve, we could not determine if the results were caused by differences in habitat or inherent to the host species. Therefore, our results only provide the first record of parasites in these two species. The findings revealed that the parasite species varied between the two areas (or species), although there was no significant difference in parasite loads.
Keywords: Aonyx capensis, Hydrictis maculicollis, Africa, parasite, urban habitat.
INTRODUCTION
Parasitism is a phenomenon found in all animals and can have consequences of different severity, from harmless to fatal. Many parasites are found in mammal hosts at the adult and reproductive stages. Parasites can profoundly impact the ecology, economy, and even human health, and the emergence and persistence of infectious diseases in wildlife pose a growing concern to public health, veterinary health, and conservation efforts (Lambert et al., 2018).
The most common parasites in the intestinal tracts of mammals are Trematoda, Cestoda, Nematoda, and Acanthocephala (Kimber and Kollias, 2000). Trematoda are one of the most common types of infectious worms and are usually contracted by eating food containing metacercariae (Toledo et al., 2014). The natural sources of trematode infections are freshwater fish, snails, and invertebrates. Parasites move from these sources to their reservoir hosts, which often are one of various mammal species (Chai and Lee, 2002). Cestodes are a group of parasites found in many mammals (Kimber and Kollias, 2000; Bartoszewicz et al., 2008) and are harmless in that they do not cause disease in most mammals. Two cestode tapeworm species (Taenia solium and Taenia saginata) regularly infect animals and are shared between domestic and wild animals (Kimber and Kollias, 2000).
River otters are carnivorous animals belonging to the Mustelidae family. They favour freshwater habitats such as rivers, lakes and riparian habitats (Kruuk, 2006). Two species of freshwater otters in South Africa are the African clawless otter (Aonyx capensis) and the spotted-necked otter (Hydrictis maculicollis). The African clawless otter is the largest (10-18 kg) otter species in the country, with males being larger than females (Somers and Nel, 2004). This species is usually found along rivers, dams, and the coastline and is active during the early morning hours, late afternoons, and early evenings (Rowe-Rowe, 1978; Somers and Purves, 1996; Somers and Nel, 2004). Their diet mainly consists of freshwater crabs, fish, frogs, and aquatic insects. Other trace food elements such as dung, molluscs, reptiles, and even birds have been reported (Rowe-Rowe, 1977; Somers and Purves, 1996; Somers and Nel, 2003; Rowe-Rowe and Somers, 1998; Jordaan et al., 2019).
Spotted-necked otters live along inland rivers, streams, and dams (Rowe-Rowe and Somers, 1998). They are a smaller species, weighing 4-6 kg (Rowe-Rowe and Somers 1998). They are primarily diurnal and prefer to be active in the early morning hours before sunrise and immediately after sunset (Rowe-Rowe, 1978; Somers and Purves, 1996). They usually feed on smaller fish, such as cichlids, followed by freshwater crabs. Amphibians, insects, and molluscs also form part of the diet, but to a lesser extent than in African clawless otters (Rowe-Rowe, 1977; Somers and Purves, 1996; Perrin and Carugati, 2000; Jordaan et al., 2020).
Prey items of the two otter species can serve as reservoirs for parasites that can be transmitted to the otters. Furthermore, the overlap of these two species’ diets may indicate that parasites could be transferred between the two species because food resources are the main causes of parasite infections. The distribution of the two species also overlaps considerably in South Africa (Rowe-Rowe and Somers, 1998).
No peer-reviewed papers on the parasites of African otters were found while preparing this work. Despite this, it is important to investigate and understand the diversity and specificity of parasites in African otters for several reasons. Firstly, African otters play a crucial role in the ecosystem as top predators, and any impacts on their health and well-being could have cascading effects on the entire ecosystem. Secondly, African otters are often found near human settlements (Somers and Nel, 2004), increasing the potential for zoonotic transmission of parasites. Therefore, understanding and managing African otters’ parasites is essential for ecological and public health reasons.
Studies conducted in Europe have shown that parasites from the four common orders (Trematoda, Cestoda, Nematoda and Acanthocephala) are prevalent in Eurasian otters (Lutra lutra) in varying numbers (Fahmy, 1954; Torres et al., 2001). One species of Trematode, Phagicola sp., was found in Eurasian otters and domestic dogs (Canis familiaris) (Chieffi et al., 1990), showing a parasite overlap between otters and domestic animals.
The three most common helminth species found in Eurasian otters are Aonchotheca putorii, Eucoleus schvalovoj, and Strongyloides lutrae (Torres et al., 2004), which were all third-stage larvae at the time of discovery. Aonchotheca putorii is a nematode usually found in the stomach of carnivores (Curtsinger et al., 1993) and has been found to cause gastritis as well as peptic ulcers in some species, such as the domestic cat (Felis catus) (Curtsinger et al., 1993). This parasitic nematode is also present in many small carnivores worldwide, such as the red fox (Vulpes vulpes) (Magi et al., 2015), and is frequently found in mustelids such as the Eurasian badger (Meles meles) (Torres et al., 2001) and the stone marten (Martes foina) (Ribas et al., 2018). Fully embryonated eggs are the main mode of infection in these species (Torres et al., 2001). The second most prevalent helminth, Strongyloides lutrae, usually only found in mustelid species, has not been reported to cause any clinical diseases in otters (Kimber and Kollias, 2000).
As African clawless otters can have large home ranges (up to 54.1 km of river for an adult male) (Somers and Nel, 2004), many are likely exposed to parasites in both rural environments (from cattle, sheep, and other wildlife) and urban environments (from cats, dogs, rats, etc.). Otters in urban areas are also likely to have increased stress levels because of their attempts to adapt their behaviour to mitigate or avoid stressors (Ditchkoff et al., 2006; Majelantle et al., 2020). Increased stress levels cause the immune system to weaken and can cause animals to come into contact with parasites they would normally not (Bradley and Altizer, 2007).
By using scat sample collection and parasite analysis, it can be determined what the prevalent species or groups of endoparasites are in otter hosts, as well as the load of these parasites (Flores et al., 2020). This helps determine how urban areas affect the health of otters in terms of parasite load. Previous studies have used these methods in urban areas to determine the prevalence of helminth species in domestic and stray dogs.
This study aimed to determine whether there is a difference in parasite loads between the two African otter species and between two different habitat types, urban and rural.
MATERIALS AND METHODS
Study Area
We collected otter spraints from two sites. One was Telperion Nature Reserve (25.703685°S, 28.941880°E), situated on the border of Gauteng and Mpumalanga provinces in South Africa. The Wilge River flows through the reserve and was the main sampling site for this study. Starting from the bridge area near the entrance to the Ezemvelo Nature Reserve (situated on the border of Telperion Nature Reserve), the area upstream and downstream was searched for latrine sites. The entire length of the drainage lines was also searched for latrine sites. Fresh otter spraints were collected from the 17th to 20th of April 2018 at Telperion Nature Reserve. The second site was in the Lynwood suburb of Pretoria, a city in South Africa. This was in the Struben Dam Bird Sanctuary (25.775792°S, 28.278548°E). A search along the entire circumference of the dam for latrine sites was conducted.
Sample Collection
At each site, latrines were identified from the size of the faecal matter, its colour, and basic composition. African clawless otter latrines were identified by the larger size of the faecal matter and the large number of crab shells in the faecal matter. Spotted-necked otter latrines were identified by the small size of the faecal matter and the presence of an anal gel in the sample. These samples also contained few crab shells compared with the African clawless otter. Faecal samples were collected from these sites, placed in sealable plastic containers, and transferred into formalin (10% buffered concentration). Samples were stored in a cool environment until identification. Formalin allows eggs, oocysts, larvae, and spores to be fixed and no longer be infectious. Twelve samples were collected from each site.
Parasite Identification
The samples were prepared for identification using Teleman’s concentration-sedimentation formalin/ether method (Rubel and Wisnivesky, 2005). Accordingly, we mixed 1 g samples with 10 ml saline solution (0.85% w/v NaCl) to wash the samples. This was then centrifuged for 10 min at a relative centrifugal force (RCF) of 600 g (about 2000 rpm). The contents were decanted, and the process was repeated until the supernatant was clear. Samples of 1 g are recommended for use with this method to ensure that the chemicals penetrate and mix thoroughly with all the chemicals. Then, 10 ml of formalin (10% buffered) and 2 ml of aether were added. This was then centrifuged for 10 min at 450 g RCF (about 1500 rpm) to separate the debris from sedimentation of faecal matter that contained eggs, oocysts, larvae, and spores. The sedimentation of the sample was then combined with saline solution and stained with iodine before being placed on viewing slides. From the viewing slides, the parasites were identified, broadly categorised into orders, and then identified at the species level. All species were identified using Thienpont et al. (1979). Eggs, oocysts, larvae, and spores were counted and measured in parasites (eggs) per gram of faeces.
Statistical Analysis
All data were analysed using R version 3.4.3 (R Core Team, 2013). The data were analysed by running a Shapiro-Wilk statistical test to determine if the data were normally distributed. We then used a Kruskal-Wallis test to determine if there were significant differences in parasite loads between sites. A Shannon diversity index was used to determine the alpha diversity of each site.
RESULTS
We analysed 24 otter faecal samples from the two species. We found 12 African clawless otter samples at Telperion Nature Reserve and 12 spotted-necked otter samples at Struben Dam Bird Sanctuary in Pretoria. Therefore, we could not compare species at each site, as was one of our initial aims.
The samples contained various parasite species in differing numbers (Appendix 1). We found that more samples had parasites than samples that did not, with 19 containing parasites and five not. Although most samples contained parasites, some contained only one or two species. The Pretoria samples contained two identified species, and the Telperion samples contained six (Figure 1; Appendix 1).
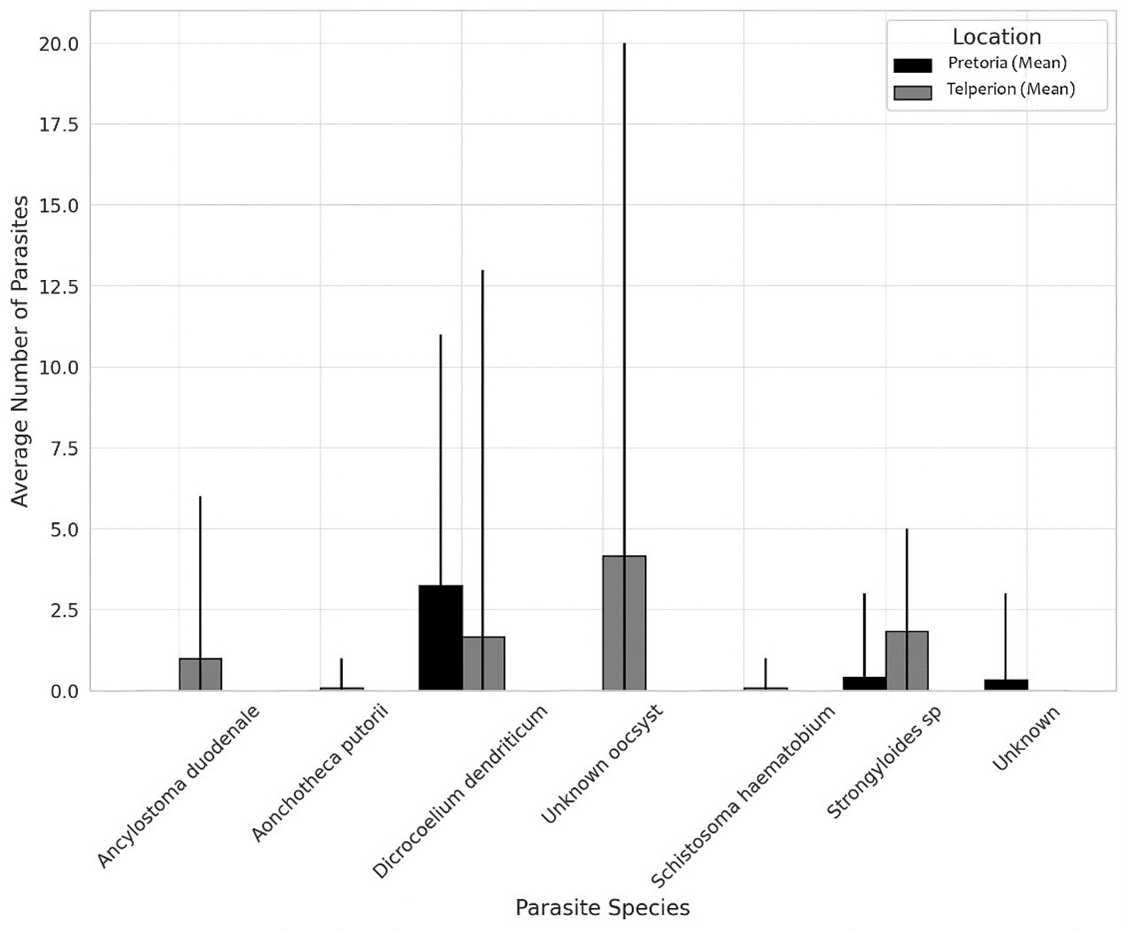
Ancylostoma duodenale was the most prevalent in Telperion, with some samples having a high count (up to 6). This parasite was absent in samples from Pretoria. Aonchotheca putorii was almost absent in both sites, with just one occurrence from Telperion. Dicrocoelium dendriticum was more common in Pretoria, where it reached counts as high as 11, compared to a maximum count of 13 at Telperion, but with fewer occurrences. The unknown oocyst was only recorded from Telprion, with some samples having a high count (up to 20). Schistosoma haematobium had just one occurrence from Telerion. Strongyloides sp were more prevalent at Telperion, with counts ranging up to 5, while at Pretoria, it reached a maximum count of 3 but was generally less prevalent. The unknown samples were only found in Pretoria, with a maximum count of 3 (Figure 1; Appendix 1).
As most species failed the normality test (P<0.05), we ran the non-parametric Kruskal-Wallis and found there was no significant difference in the number of parasites between sites (Kruskal-Wallis χ 2=10.443, df=23, P=0.4911) and, therefore, between otter species. The alpha diversity of the two areas presented a Shannon diversity index =1.3268 for Telperion compared with that for the Pretoria site =0.3095.
DISCUSSION
The recording of only African clawless otter samples in the nature reserve and spotted-necked otter samples in the urban reserve confounded our comparison between urban and natural habitats. Therefore, our results are only the first record of parasites in these two species. The differences could be due to differences in species or habitat.
Overall, we found that more faecal samples contained intestinal parasites than samples that did not. The samples from the urban and natural areas contained varying numbers of parasite eggs, with the natural area having the highest number on average. However, this difference was not significant.
The samples from Telperion Nature Reserve were found to have higher species diversity than those from the Pretoria area. Collective findings suggest that parasite diversity may be higher in natural habitats than in disturbed urban habitats (see Werner and Nunn, 2020). This is expected because parasites have complex life histories, and urban, disturbed environments have a greater potential to disrupt one of the parasite’s life stages. The natural area otters also potentially have access to a greater diversity of other mammals (Webster et al., 2021), from which they could be exposed to a wider variety of parasite species (McKinney, 2002).
Most of the Pretoria green belt reserves are connected, but this connection is mostly in the form of a small stream that also moves through urban areas and pipes, thus not permitting the movement of animals, including domestic livestock, that might be parasite hosts. Not all parasites are obtained from external reservoirs, though, as some are spread from mother to offspring (Carlier and Truyens, 1995) and within the otter community through the use of communal latrines (Ben-David et al., 2005).
Dietary differences between species may also explain the recorded differences in parasite loads (e.g., Woodstock et al., 2020). African clawless otters potentially have a broader dietary niche than spotted-necked otters, and this could therefore be the cause of the higher species diversity of parasites.
Urban parks such as Struben Dam Bird Sanctuary are often used as recreational areas by humans and their pets (Miller et al., 2001). These pets include dogs that are taken for exercise in the parks and cats that roam into these areas from neighbouring houses. These domestic species can come into contact with otter faecal matter and distribute their own faecal matter. This causes a flow of parasites between domestic and wild species. Liccioli et al. (2012) found that coyotes in urban areas create a parasite flow between them and domestic dogs. Protected natural areas such as Struben Dam Bird Sanctuary are fenced-off areas that fall within the habitat range of otter species (Rowe-Rowe and Somers, 1998). Otters can spread parasites into these areas and contract parasites from contact with domestic pets in these residential areas. If parasites spread to domestic animals, the spread of infection may also affect human health. Animals that use latrine areas, such as otters, deposit faeces in open areas, which can furthermore be a risk for children visiting such areas (Bateman and Fleming, 2012). Small children are likely to dig around in the dirt and accidentally find a latrine where they can get infected with parasites. Parasites found in the Struben Dam samples have been found to infect humans and cause disease symptoms.
Sewage runoff, which is common in South Africa, is a problem in other urban areas. The spread of human and domestic animal parasites to otters due to increased farming and sewage runoff might also increase in the near future, with water pollution becoming an increasing problem in South Africa (Madilonga et al., 2021). In California, USA, it was found that sewage runoff into coastal rivers and dams caused otters to get infected with parasites that their immune system was not strong enough to fight (Miller et al., 2010). One such parasite, Toxoplasma gondii, a protozoan parasite commonly found in cat faecal matter, was present in human sewage runoff and has caused many casualties in the population (Miller et al., 2008). This shows that domestic parasites can be much more harmful to the otter community than otter parasites.
Dicrocoelium dendriticum are parasites usually found in cattle and sheep in agricultural areas (González-Lanza et al., 2003). The parasite also has an intermediate host of molluscs followed by ants, which are ultimately consumed by cattle and sheep (González-Lanza et al., 2003). The otters may be merely accidental hosts as they eat molluscs (Rowe-Rowe, 1978; Rowe-Rowe and Somers, 1998). Therefore, it may be possible that otters do not aid in the spread of this parasite because critical development of the parasite cannot occur in the otter host but must occur in another host (González-Lanza et al., 2003). This parasite likely spread to the Telperion Nature Reserve because the surrounding area contains cattle and sheep farms. Otters can also move great distances because their home ranges can be large (Somers and Nel, 2004), which might cause them to come into contact with cattle and sheep outside Telperion Nature Reserve.
The unidentified parasite oocyst was the second most prevalent parasite and could not be identified because many oocysts have a similar structure at this stage of development. The oocysts from this study are structurally similar and form part of the same species. The oocyst is the last stage of development for many species and is usually not used for identification. More developed forms, such as breeding adults, are found in the rest of the body, including the gastrointestinal tract, and are usually used for identification. To correctly identify oocysts in a faecal examination, specific staining techniques must be applied to correctly visualise the correct structures (Arrowood and Sterling, 1989). These in-depth methods were not applied as they were beyond the scope of the study and could be applied in future studies building on what has already been done in this study.
Strongyloides species were present in the samples and were found at both sites. Strongyloides species are closely related in structure, and it can be difficult to identify these parasites up to the species level. The species present in the samples were Strongyloides lutrae as this species has been found in otters by previous studies and is the most common species found in mustelids (Kimber and Kollias, 2000).
Ancylostoma species have been found in a wide variety of species, including otters (Torres et al., 2004). In urban areas, it has been found in domestic canids and domestic and feral cats (Barutzki and Schaper, 2011; Mackenstedt et al., 2015). Ancylostoma species also harm humans and can form parasite-filled lesions all over the body if the infection becomes severe (Mackenstedt et al., 2015).
Schistosoma haematobium usually occurs in freshwater snails (Young et al., 2012). The snails are intermediate hosts where development occurs before they move to humans, which are their definitive hosts (Young et al., 2012). This parasite was only found once. Otters are unlikely to be an intermediate or definitive host for this species, and this was thus perhaps an accidental infection caused by otters eating freshwater snails as part of their diet (Rowe-Rowe and Somers, 1998).
CONCLUSION
The study can be seen as a first record. Future work would benefit from a larger sample size of habitats and sites and faeces collected from areas with both species. We also suggest using a sedimentation method in the future that is not as sensitive to centrifugal pressure. Examining the relationship between water quality and parasite loads would further be an interesting avenue of research. We hope that this note will stimulate a more widespread study on the parasites and their role in the ecology, health, and conservation of African otters.
In summary, while this study has provided crucial initial data on gastrointestinal parasites in South African otters, comprehensive and continued surveillance is needed to better understand, monitor, and control the spread and impact of these parasites.
Acknowledgements: We thank E Oppenheimer & Son Pty Ltd. and Duncan McFadyen for permission to work on their property. We thank the Eugène Marais Chair of Wildlife Management for funding. Morné Oosthuysen is thanked for assisting in the fieldwork. ABEERU: University of South Africa is thanked for providing accommodation during fieldwork.
REFERENCES
Arrowood, M., Sterling, C. (1989). Comparison of conventional staining methods and monoclonal antibody-based methods for cryptosporidium oocyst detection. J. Clin. Microbiol. 27: 1490-1495. https://doi.org/10.1128/jcm.27.7.1490-1495.1989
Bartoszewicz, M., Okarma, H., Zalewski, A., Szczęsna, J. (2008). Ecology of the raccoon (Procyon lotor) from Western Poland. Ann. Zool. Fenn. 45: 291-298. https://doi.org/10.5735/086.045.0409
Barutzki, D., Schaper, R. (2011). Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol. Res. 109: 45-60. https://doi.org/10.1007/s00436-011-2402-8
Bateman, P., Fleming, P. (2012). Big city life: carnivores in urban environments. J. Zool. 287: 1-23. https://doi.org/10.1111/j.1469-7998.2011.00887.x
Ben-David, M., Blundell, G., Kern, J., Maier, J., Brown, E., Jewett, S. (2005). Communication in river otters: creation of variable resource sheds for terrestrial communities. Ecology 86: 1331-1345. https://doi.org/10.1890/04-0783
Bradley, C., Altizer, S. (2007). Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 22: 95-102. https://doi.org/10.1016/j.tree.2006.11.001
Carlier, Y., Truyens, C. (1995). Influence of maternal infection on offspring resistance towards parasites. Parasitol. Today 11: 94-99. https://doi.org/10.1016/0169-4758(95)80165-0
Chai, J., Lee, S. (2002). Food-borne intestinal trematode infections in the Republic of Korea. Parasitol. Int. 51: 129-154. https://doi.org/10.1016/S1383-5769(02)00008-9
Chieffi, P., Leite O., Dias, R., Torres, D., Mangini, A. (1990). Human parasitism by Phagicola sp (Trematoda, Heterophyidae) in Cananéia, São Paulo State, Brazil. Rev. Inst. Med. Trop. Sp. 32: 285-288. https://doi.org/10.1590/S0036-46651990000400008
Curtsinger, D., Carpenter, J., Turner, J. (1993). Gastritis caused by Aonchotheca putorii in a domestic cat. J. Am. Vet. Med. A. 203: 1153-1154. https://doi.org/10.2460/javma.1993.203.08.1153
Ditchkoff, S., Saalfeld, S., Gibson, C. (2006). Animal behaviour in urban ecosystems: Modifications due to human-induced stress. Urban Ecosyst. 9: 5-12. https://doi.org/10.1007/s11252-006-3262-3
Fahmy, M. (1954). On some helminth parasites of the otter, Lutra lutra. J. Helminthol. 28: 189-194. https://doi.org/10.1017/S0022149X00032867
Flores, E.Y.B., Torres Tobón, M.G., Cabrera, C.G.A., Ramírez-Bravo, O.E., Callejas, E.R. (2020). Gastrointestinal parasites in the neotropical otter (Lontra longicaudis annectens) in Central Mexico. West. N. Am. Nat. 80: 540–542. https://doi.org/10.3398/064.080.0412
González-Lanza, C., Manga-González, M., Cabanas, E., Campo, R. (2003). Contributions to and review of dicrocoeliosis, with special reference to the intermediate hosts of Dicrocoelium dendriticum. Parasitology 123: 146-149. https://doi.org/10.1017/S0031182001008204
Jordaan, R.K., Somers, M.J., Hall, G., McIntyre, T. (2019). Plasticity and specialisation in the isotopic niche of African clawless otters foraging in marine and freshwater habitats. Mamm. Biol. 98: 61–72. https://doi.org/10.1016/j.mambio.2019.07.006
Jordaan, R.K., Somers, M.J., Hall, G., McIntyre, T. (2020). The diet of spotted-necked otters foraging in trout-stocked waters in Mpumalanga, South Africa. Afr. Zool. 55: 141–148. https://doi.org/10.1080/15627020.2020.1741447
Kimber, K., Kollias, G. (2000). Infectious and parasitic diseases and contaminant-related problems of North American river otters (Lontra canadensis): a review. J. Zoo Wildlife Med. 31: 452-472. https://doi.org/10.1638/1042-7260(2000)031[0452:IAPDAC]2.0.CO;2
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780198565871.001.0001
Lambert, S., Ezanno, P., Garel, M., Gilot-Fromont, E. (2018). Demographic stochasticity drives epidemiological patterns in wildlife with implications for diseases and population management. Sci. Rep. 8: 16846. https://doi.org/10.1038/s41598-018-34623-0
Liccioli, S., Catalano, S., Kutz, S., Lejeune, M., Verocai, G., Duignan, P., Fuentealba, C., Hart, M., Ruckstuhl, K., Massolo, A. (2012). Gastrointestinal parasites of coyotes (Canis latrans) in the metropolitan area of Calgary, Alberta, Canada. Can. J. Zool. 90: 1023-1030. https://doi.org/10.1139/z2012-070
Mackenstedt, U., Jenkins, D., Romig, T. (2015). The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 4: 71-79. https://doi.org/10.1016/j.ijppaw.2015.01.006
Madilonga, R.T., Edokpayi, J.N., Volenzo, E.T., Durowoju, O.S., Odiyo, J.O. (2021). Water quality assessment and evaluation of human health risk in Mutangwi River, Limpopo province, South Africa. Int. J. Environ. Res. Public Health 18: 6765. https://doi.org/10.3390/ijerph18136765
Magi, M., Guardone, L., Prati, M., Mignone, W., Macchioni, F. (2015). Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 89: 506-511. https://doi.org/10.1017/S0022149X1400025X
Majelantle, T.L., McIntyre, T., Ganswindt, A. (2020). Monitoring the effects of land transformation on African clawless otters (Aonyx capensis) using faecal glucocorticoid metabolite concentrations as a measure of stress. Integr. Zool. 15: 293-306. https://doi.org/10.1111/1749-4877.12428
McKinney, M. (2002). Urbanization, biodiversity, and conservation. BioScience 52: 883. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
Miller, M., Conrad, P., Harris, M., Hatfield, B., Langlois, G., Jessup, D., Magargal, S., Packham, A., Toy-Choutka, S., Melli, A., Murray, M., Gulland, F., Grigg, M. (2010). A protozoal-associated epizootic impacting marine wildlife: mass-mortality of southern sea otters (Enhydra lutris nereis) due to Sarcocystis neurona infection. Vet. Parasitol. 172: 183-194. https://doi.org/10.1016/j.vetpar.2010.05.019
Miller, M., Gardner, I., Kreuder, C., Paradies, D., Worcester, K., Jessup, D., Dodd, E., Harris, M., Ames, J., Packham, A., Conrad, P. (2002). Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 32: 997-1006. https://doi.org/10.1016/S0020-7519(02)00069-3
Miller, M., Miller, W., Conrad, P., James, E., Melli, A., Leutenegger, C., Dabritz, H., Packham, A., Paradies, D., Harris, M. (2008). Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 38: 1319-1328. https://doi.org/10.1016/j.ijpara.2008.02.005
Miller, S., Knight, R., Miller, C. (2001). Wildlife responses to pedestrians and dogs. Wildl. Soc. B. 29: 124-132. https://www.jstor.org/stable/3783988
Perrin, M., Carugati, C. (2000). Food habits of coexisting Cape clawless otter and spotted-necked otter in the KwaZulu-Nata Drakensberg, South Africa. S. Afr. J. Wildl. Res. 30: 85-92. https://hdl.handle.net/10520/EJC117096
R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Ribas, A., Milazzo, C., Casanova, J. (2018). New data on helminths of stone marten, Martes foina (Carnivora, Mustelidae), in Italy. Helminthologia 41: 59-61. https://www.researchgate.net/publication/356379543
Rowe-Rowe, D.T. (1977). Food Ecology of Otters in Natal, South Africa. Oikos 28: 210–219. https://doi.org/10.2307/3543973
Rowe-Rowe, D.T. (1978). The small carnivores of Natal. Lammergeyer 25: 1-48.
Rowe-Rowe, D.T., Somers, M.J. (1998). Diet, foraging behaviour and coexistence of African otters and the water mongoose. In: Dunstone, N., Gorman, M.L., (Eds). Behaviour and Ecology of Riparian Mammals. Symposia of the Zoological Society of London, 71. Cambridge University Press; 1998: pp 215-228. https://doi.org/10.1017/CBO9780511721830.014
Rubel, D., Wisnivesky, C. (2005). Magnitude and distribution of canine faecal contamination and helminth eggs in two areas of different urban structure, Greater Buenos Aires, Argentina. Vet. Parasit. 133: 339-347. https://doi.org/10.1016/j.vetpar.2005.06.002
Somers, M.J., Nel, J.A.J. (2003). Diet in relation to prey of Cape Clawless otters in two rivers in the Western Cape Province, South Africa. Afr. Zool. 38(2): 317-326. https://hdl.handle.net/10520/EJC17881
Somers, M.J., Nel, J.A.J. (2004). Movement patterns and home range of Cape clawless otters (Aonyx capensis), affected by high food density patches. J. Zool. 262: 91-98. https://doi.org/10.1017/S095283690300445X
Somers, M.J., Purves, M.G. (1996). Trophic overlap between three syntopic semi-aquatic carnivores: Cape clawless otter, spotted-necked otter and water mongoose. Afr. J. Ecol. 34: 158-166. https://doi.org/10.1111/j.1365-2028.1996.018-89018.x
Thienpont, D., Rochette, F., Vanparijs, O. (1979). Diagnosing helminthiasis through coprological examination. 1st edition. Janssen Research Foundation, Beerse. https://www.researchgate.net/publication/283924775
Toledo, R., Muñoz-Antoli, C., Esteban, J. (2014). Intestinal trematode infections. Adv. Exp. Med. Biol. 766: 201-240. https://doi.org/10.1007/978-1-4939-0915-5_7
Torres, J., Miquel, J., Motjé, M. (2001). Helminth parasites of the Eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol. Res. 87: 259-263. https://doi.org/10.1007/s004360000316
Torres, J., Torres, J., Feliu, C., Fernández-Morán, J., Ruíz-Olmo, J., Rosoux, R., Santos-Reis, M., Miquel, J., Fons, R. (2004). Helminth parasites of the Eurasian otter Lutra lutra in southwest Europe. J. Helminthol. 78: 353-359. https://doi.org/10.1079/JOH2004253
Webster, A.B., Pretorius, M.E., Somers, M.J. (2021). The determinants of mesocarnivore activity patterns in highveld grassland and riparian habitats. Afr. J. Wildl. Res. 51: 178–192. https://repository.up.ac.za/bitstream/handle/2263/85928/Webster_Determinants_2021.pdf?sequence=1&isAllowed=y
Werner, C.S., Nunn, C.L. (2020). Effect of urban habitat use on parasitism in mammals: a meta-analysis. Proc. Biol. Sci. 287: 20200397. https://doi.org/10.1098/rspb.2020.0397
Woodstock, M.S., Blanar, C.A., Sutton, T.T. (2020). Diet and parasites of a mesopelagic fish assemblage in the Gulf of Mexico. Mar. Biol. 167: 184. https://doi.org/10.1007/s00227-020-03796-6
Young, N., Jex, A., Li, B., Liu, S., Yang, L., Xiong, Z., Li, Y., Cantacessi, C., Hall, R., Xu, X., Chen, F., Wu, X., Zerlotini, A., Oliveira, G., Hofmann, A., Zhang, G., Fang, X., Kang, Y., Campbell, B., Loukas, A., Ranganathan, S., Rollinson, D., Rinaldi, G., Brindley, P., Yang, H., Wang, J., Wang, J., Gasser, R. (2012). Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 44: 221-225. https://doi.org/10.1038/ng.1065
Parasite species found in the faecal samples of two otter species, the African clawless otter (AC) and the spotted-necked (SN) otter. The number of each parasite species per faecal sample is indicated, as well as the otter species to which the sample belongs.
| Sample Number | Otter Species | Parasite Species Name | ||||||
| Aonchotheca putorii | Strongyloides sp | Ancylostoma duodenale | Unknown oocyst | Dicrocoelium dendriticum | Schistosoma haematobium | Unknown | ||
| 1 | AC | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | AC | 0 | 3 | 0 | 0 | 0 | 1 | 0 |
| 3 | AC | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | AC | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 5 | AC | 0 | 2 | 4 | 5 | 0 | 0 | 0 |
| 6 | AC | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| 7 | AC | 0 | 1 | 1 | 10 | 0 | 0 | 0 |
| 8 | AC | 0 | 5 | 0 | 15 | 0 | 0 | 0 |
| 9 | AC | 0 | 3 | 0 | 20 | 13 | 0 | 0 |
| 10 | AC | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | AC | 0 | 1 | 0 | 0 | 7 | 0 | 0 |
| 12 | AC | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 13 | SN | 0 | 3 | 0 | 0 | 10 | 0 | 0 |
| 14 | SN | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 15 | SN | 0 | 0 | 0 | 0 | 11 | 0 | 0 |
| 16 | SN | 0 | 1 | 0 | 0 | 10 | 0 | 0 |
| 17 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 18 | SN | 0 | 0 | 0 | 0 | 2 | 0 | 3 |
| 19 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 22 | SN | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| 23 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | SN | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Résumé: Première Observation de Parasites Gastro-Intestinaux de Loutres Africaines
Les parasites sont présents chez de nombreuses espèces de mammifères, notamment dans le tractus gastro-intestinal. Ils peuvent se propager d’une espèce à l’autre et provoquer de graves maladies chez certaines espèces. La propagation des parasites est particulièrement importante entre les animaux sauvages et domestiques car elle peut affecter la santé humaine. Dans cette étude, la première observation de parasites gastro-intestinaux chez des loutres africaines est signalée en Afrique du Sud. Les deux espèces de loutres étudiées étaient la loutre africaine à joues blanches (Aonyx capensis) et la loutre à cou tacheté (Hydrictis maculicollis). Nous avons identifié les espèces de parasites dans les excréments collectés et examiné les différences de charges parasitaires entre les espèces et deux habitats distincts. Les excréments ont été prélevés sur des sites de latrines dans deux zones (une réserve naturelle rurale et un parc urbain) et examinés à l’aide de la méthode concentration-sédimentation formol/éther de Teleman. Les parasites ont été identifiés jusqu’au niveau de l’espèce, lorsque c’était possible et les charges calculées. Comme nous n’avons trouvé que des excréments de loutres africaines à joues blanches dans la zone naturelle et des excréments de loutres à cou tacheté dans la réserve urbaine, nous n’avons pas pu déterminer si les résultats étaient causés par des différences d’habitat ou inhérents à l’espèce hôte. Par conséquent, nos résultats ne fournissent que la première observation de parasites chez ces deux espèces. Les résultats ont révélé que les espèces de parasites variaient entre les deux zones (ou espèces), bien qu’il n’y ait pas de différence significative dans les charges parasitaires.
Revenez au dessus
Resumen: Primer Registro de Parásitos Gastrointestinales de Nutrias Africanas
Se encuentran parásitos en muchas especies de mamíferos, particularmente en el tracto gastrointestinal. Se pueden dispersar de una especie a otra y causar enfermedades severas en algunas especies. La dispersión de parásitos es especialmente importante entre animales silvestres y domésticos, porque puede afectar la salud humana. En este estudio, se informa del primer registro de parásitos gastrointestinales en nutrias Africanas, en Sudáfrica. Las dos especies de nutrias investigadas fueron la nutria sin garras Africana (Aonyx capensis) y la nutria de cuello manchado (Hydrictis maculicollis). Identificamos la especie de parásito en fecas colectadas y examinamos las diferencias en las cargas parasitarias entre las especies y entre dos hábitats distintos. Colectamos fecas de sitios de letrinas en dos áreas (una reserva natural rural y un parque urbano) y las examinamos utilizando el método de Teleman de concentración-sedimentación con formalina/éter. Los parásitos fueron identificados hasta el nivel de especie cuando fue posible, y se calcularon las cargas. Como en el área natural encontramos solamente fecas de nutria sin garras Africana, y en la reserva urbana solamente de nutria de cuello manchado, no pudimos determinar si los resultados fueron causados por diferencias en el hábitat, o por factores inherentes a las especies huésped. por lo tanto, nuestros resultados solamente proporcionan el primer registro de parásitos en estas dos especies. Los hallazgos revelaron que las especies de parásito variaron entre ambas áreas (o especies), aunque no hubo diferencia significativa en las cargas parasitarias..
Vuelva a la tapa