IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 41 Issue 5 (December 2024)
Citation: Narasimmarajan, K., Mathai, M.T., Hayward, M.W. and Palanivel, S. (2024). Lesser-Known Sentinels: Role of Environmental Variables influencing the Seasonal Resource Use Patterns of Asian Small-Clawed Otters (Aonyx cinereus nirnai) in the Western Ghats Moyar River Biodiversity Hotspots. IUCN Otter Spec. Group Bull. 41 (5): 296 – 310
Lesser-Known Sentinels: Role of Environmental Variables influencing the Seasonal Resource Use Patterns of Asian Small-Clawed Otters (Aonyx cinereus nirnai) in the Western Ghats Moyar River Biodiversity Hotspots.
Kannadasan Narasimmarajan1,2*, Manu Thomas Mathai1, Matthew W. Hayward3, and Sonaimuthu Palanivel4
1Dept. of Zoology, Madras Christian College, Tambaram, Chennai – 600059, India
2Bombay Natural History Society, Hornbill House, Opp. Lion Gate, Shaheed Bhagat Singh Marg, Fort,
Mumbai – 400001, India
3Conservation Science Research Group, University of Newcastle, Callaghan, NSW 2308, Australia
4G and Research Department of Biotechnology, Sri Sankara Arts and Science College (Affiliated to University of Madras), Kanchipuram - 631561, Tamil Nadu, India
*Corresponding Author Email: wildlife9protect@gmail.com
Received 16th June 2024, accepted 30th September 2024
Abstract:We examined the role of environmental variables influencing the resource use patterns of Asian small-clawed otters (Aonyx cinereus nirnai) by sampling the entire River Moyar of the Western Ghats between March 2015 and September 2017, using otter signs as an indicator. An occurrence-based framework was used to determine the influence of environmental covariates on otter detectability. Information on environmental parameters was recorded every time otter signs were detected and non-detected at sites spaced every 400 meters along the riverbanks in the post monsoon, winter, and summer seasons. Detectability of otter sign was influenced by river substrate, habitat characteristics, riverbank traits and forest types. Otters prefer high altitude/elevation, narrow rivers, and rocky areas with shallow water, but avoided sandy, wider and deep river areas. Resource use patterns were determined by river and habitat characteristics in all three seasons. Various forms of disturbance adversely affected otter occurrence. Asian small-clawed otters required habitat specific specialized environmental traits for their long-term endurance in human-dominated landscape. Restoration of degraded habitats and sites invaded by non-indigenous wattle trees is necessary to improve the long-term conservation prospects of the Asian small-clawed otter. Otter conservation plans need to be species-specific to help maintain the ecological balance of the Moyar River ecosystem.
Keywords: Asian small-clawed otters; Conservation; Environmental variables; Resource use patterns; habitat traits; Western Ghats
INTRODUCTION
The Aonyx cinereus nirnai Illiger, 1815 is a recognized subspecies of the Asian small-clawed otter - the smallest otter of the world occurring in the Western Ghats region, Southern India (Narasimmarajan, 2020; Hussain, 1999) where it is restricted to a few hill streams in the region (Perinchery et al., 2011). Being an amphibious 'apex predator' of the aquatic ecosystem otters play an important role for land-water continuum (Narasimmarajan et al., 2023) and the otter presence is largely dependent on the continuous availability of adequate prey base and uncontaminated aquatic environments (Melquist and Hornocker, 1983; Macdonald and Mason, 1983). Most wetlands and waterways in Asia, however, lack an adequate prey base for sustaining otter populations as a result of pollution by eutrophication and the accumulation of pesticide runoff into the water, poaching for the pelt and pet trade, hydroelectric dams, illegal hunting, ichthyotoxic plants, indiscriminate fishing, and habitat degradation (Wright et al., 2015; Narasimmarajan et al., 2021). Thus, the disappearance of otters from apparently suitable sites is often associated with the habitat degradation and natural deaths in river pits of their wetland habitats (Narasimmarajan et al., 2024) and human causes such as hunting (Kruuk, 1995).
The Asian small-clawed otter is widespread from India through southern Asia, and it is the smallest otter species in the world (Hussain, 1999). The Asian small-clawed otter is considered ‘Vulnerable’ to extinction by the IUCN Red list A2cde+3cde ver 3.1 (Wright et al., 2021). Increased influx of pesticides into the streams from the plantations reduces the quality of their habitats. The threat posed by poaching is still very significant in many parts of India, and South-east Asia and will certainly count as a major threat that needs to be constantly monitored. Poaching for pelts has been reported from across the Western Ghats in southern India (Meena, 2002; Prakash et al., 2012). The Asian small-clawed otter is currently under-represented in the literature, which may partly be due to difficulty in documenting them in their natural habitat (Narasimmarajan, 2020; Prakash et al., 2012; Perinchery et al., 2011).
Only a few detailed Asian small-clawed otter survey reports exist in India (Perinchery et al., 2011; Prakash et al., 2012; Mohapatra et al., 2014; Raha and Hussain, 2016; Narasimmarajan, 2020; Palei et al., 2023), which point to a preference for high hill streams (Perinchery et al., 2011). These records contribute to our understanding of the coarse habitat selection by Aonyx cinereus; however, fine-scale patterns of habitat selection in this preferred hilly terrain remain poorly understood. Species assessments predict a decreasing population trend due to habitat loss and conversion; with a diet composed chiefly of crabs, crustaceans, and other molluscs (Sivasothi and Nor, 1994). Asian small-clawed otters prefer moderate to low vegetation structure (possibly for escape cover) in riparian systems, although they also have been recorded from areas with sparse vegetation (Hussain and da Silva., 2008). Other records of Asian small-clawed otters are from peat swamps, rice fields, and other brackish and marine habitats in Malaysia (Sivasothi and Nor, 1994). We aimed to identify the environmental factors that influence the resource use patterns of Asian small-clawed otters in the Moyar River, southern Western Ghats of southern India. We attempted to develop detailed data on factors that influenced the species’ persistence in protected habitats, however future studies should focus on anthropogenic changes in otter habitats and the consequences of this to survival of otter populations over different seasons.
MATERIALS AND METHODS
Study Area
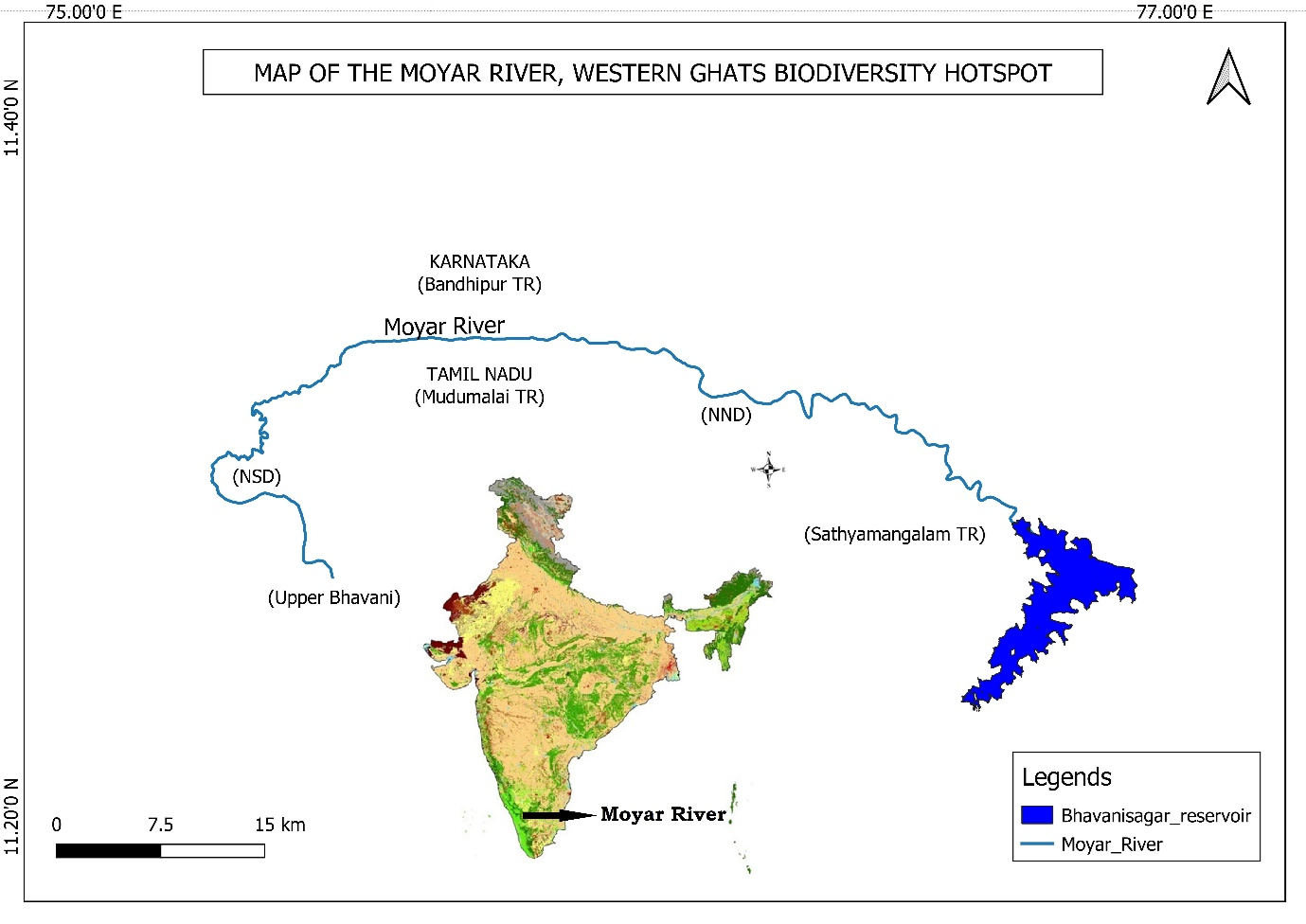
The Moyar River is 102 km long and is located within the UNESCO recognized world heritage site of the Nilgiri Biosphere Reserve. The river originates in Upper Bhavani at 2054 masl in the Nilgiri district of Tamil Nadu, India, and then flows through several protected areas (Mudumalai and Sathyamangalam Tiger Reserves, Nilgiri North and South Forests Divisions), and ends in Bavanisagar Dam at 254 masl in Erode District (Fig. 1) (Narasimmarajan et al., 2018). About 47 km of the Moyar River borders the Bandipur Tiger Reserve, Karnataka. The upper reaches of the river area receive >5,000 mm of rainfall, whereas the downstream area receives ~824 mm of rainfall annually (Puyravaud and Davidar, 2013). The minimum and maximum annual average temperatures in this region vary from 14 °C - 30 °C in higher elevations, and 25 °C - 38 °C in the lower elevations (Narasimmarajan et al., 2018). The Mudumalai, Sathyamangalam, Bandipur landscape supports a large population of Tiger (Panthera tigris), Leopard (Panthera pardus), Asian elephant (Elephas maximus), Otters (Lutrogale perspicillata; Aonyx cinereus), Dhole (Cuon alpinus), and Endangered Vultures (Gyps benghalensis; Gyps indicus) (Narasimmarajan et al., 2021).
The riverbanks of the Moyar support different forest types such as evergreen forests, riparian forests, deciduous forests, scrub forests, bamboo dominated riparian forests and non-indigenous invasive plants including black wattle (Acacia melanoxylon), mesquite (Prosopis juliflora) (introduced by British colonial era for firewood) and Lantana camara that is catastrophically invading the river gorges (Champion and Seth, 1962; Narasimmarajan et al., 2021).
The Moyar River is an important source of irrigation for thousands of hectares of agricultural land and supports the livelihoods of more than a million people (Puyravaud and Davidar, 2013). However, like other freshwater river ecosystems in India, the River Moyar faces many threats, such as agricultural pesticide runoff, hydroelectric projects, unrestricted illegal fishing activities, invasive species, and the spilling of motor oil (Narasimmarajan 2020).
Data Collection
The data were collected between March 2015 and September 2017. We aimed to investigate the seasonal resource use patterns of Asian small-clawed otters in the Moyar River study region using the survey methods described by Hussain and Choudhury (1995); Nawab and Hussain (2012); and Narasimmarajan et al. (2021). The entire River Moyar and its tributaries were divided into 6 km segments based on Narasimmarajan et al. (2023) using geographical information systems. During the survey, whenever otter signs were seen, data on environmental parameters, and spraints, tracks, dens, and grooming sites were recorded in 15 m width x 100 m length strip width plots. In addition, at each significant crossing/access point where otter sign was not detected, a random stratified plot (15 x 100 m stripe width) of the Moyar River was surveyed to compare the resource use pattern of otters in latrine and non-latrine sites (Narasimmarajan et al., 2021). A team of four researchers conducted the survey by walking along both riverbanks, searching for otter signs. In each survey season (post-monsoon (September - November), winter (December - February) and summer (March - June), the plots where spraints, tracks, grooming plots, dens and other signs of otter presence were found were defined as a ‘used/positive site’. Signs are correlated positively with otter habitat use and preference (Guter et al., 2008). A new plot was considered as such only when spraints were separated by >15 m away from previous used/positive site (Melquist and Hornocker, 1983; Newman and Griffin, 1994; Medina, 1996), whereas spraints within 15 m were considered as the same latrine site. For the estimation of habitat availability, each plot was categorized as a rocky stretch, sandy stretch, muddy stretch, clayey stretch and alluvial stretch. In each survey season, data on environmental parameters (i.e., river character variables, habitat character variables and substrate variables) and disturbance that were considered potentially important to otters were collected from each plot (Macdonald and Mason, 1983; Brzeziński and Jedrzejewska, 1993; Anoop and Hussain, 2004) (Table 1).
Opportunistic observations of otters during the course of the surveys were also recorded and their group size, structure and activity were noted.
Data Analysis
A detection history was created based on whether otter signs were detected (1) or non-detected (0) at each 400 m (15 x 100 m strip width plot) along the riverbank for each season. The covariate data collected for available and used plots was organized in sample-habitat parameter matrix for post monsoon, winter and summer seasons respectively. The raw data matrix was arranged into proportionate and continuous data, which had to be transformed via arcsine and log transformation and standardized following Zar (1984). Factor analysis was used to reduce the dimensionality of the environmental variables. The first three factors (predictors) were used for interpretation as these explained maximum variations in the dataset, and Pearson product moment coefficient as the input and a varimax rotation of these factors (Van Emden, 2008). Simple cross-tabulations and χ2 statistics were used to calculate the detection of possible relationships between resource variables (i.e., the presence/absence of different environmental traits, and presence/absence of sprainting activity) and auto-correlated null variables were dropped due to their nonaligned influence on the sprainting activity (Van Emden, 2008). Pearson’s correlation was used to subset the null deviations, and the constant-only model was used to identify the significant covariates for further analysis (MacKenzie, 2006; Kruuk, 1995). Significant associations between habitat traits, and the presence or absence of spraints were enumerated
Spraints are likely to be detected more often than expected where rocks and boulders occur in the immediate vicinity of a survey plot (White et al., 2003) and may be overlooked in dense vegetation. Spraints are likely to be found less often than expected at plots with grass either in the surrounding land or the immediate vicinity respectively. Pearson’s correlation was used to subset the null deviations and constant-only model used to test the significance of the covariates of further analysis (MacKenzie, 2006). We attempted to account for this differential detectability using land cover variables representing river order, river gradient and habitat characters in the logistic regression analysis (White et al., 2003). Global logistic regression model including variables relating to habitat, the physical characteristics of the river and surrounding vegetation cover was able to predict the presence or absence of otter sprainting at different survey plots with an accuracy of 92% (Z-value) using software R (R Core Team, 2018).
RESULTS
Distribution Patterns of Asian Small-Clawed Otter Signs in the River Moyar, Western Ghats
Total of 693 strip width plots (15 m width x 100 m length) were surveyed, in which 87 Asian small-clawed otter positive/used sites were recorded. Otter signs were found at 24.7% (n=18) of sites during the post-monsoon, 34.0% (n=27) during winter and 41.4% (n=42) during summer (Table 2) consisting of 73 spraints, 11 tracks, 3 grooming site and 3 active dens. Dens were usually made under wild mango (Mangifera indica) trees in the River Moyar. Observations clearly show that the Asian small-clawed otter occurs in the Moyar river in all three seasons i.e., Post monsoon, Winter and Summer. Latrine sites were mostly found between 796 m asl to 2050 m asl in the river Moyar where the dense forests cover and narrow river with steep bank slope habitat dominated.
The logistic regression analysis showed an efficiency of 91.61% of available and utilized plots. This model also suggested that the Asian small-clawed otter sprainting activities were found in rocky areas with steep bank slopes, alongside the presence of many tall trees, and tall grass cover with less disturbance respectively.
Otter sprainting sites were found in narrow river stretches, and they avoided sprainting far from riverbanks. Otters also avoided low altitudes and high amounts of dry leaf litter cover at sites for grooming, while favouring river pits/pools in the high-altitude areas.
Otter sprainting activities were positively linked with the river pools/pits and stagnant water currents in the high-altitude areas, and the sprainting sites were not recorded in wattle invaded areas near the riverbanks. Spraints were mostly recorded at sites with more shoreline vegetation cover. Spraint sites were influenced by prey availability and otter spraints were as likely to occur near mugger crocodile sites as not (< 650 m asl). Higher otter spraints were recorded from sites that had rocky, high altitude and no disturbance areas.
Resource Availability
Fourteen categories of environmental covariates were surveyed in the study area. Overall landscape level environmental variables, such as mean hard sand (46.26%), followed by rocky stretches composed of boulders (28.19%), loose sand stretches (15.43%) and while stones constituted the least (7.01%) in the otter used sites. Other habitat variables were measured, including the mean canopy cover (51.24%), dry leaf litter cover (40.59%), riverbank grass height (2.83%). River characters represented by water current attitude (1.87) (2-slow), bank slope (steep/moderate), mean river depth (16.72 m) and river width (12.88 m) (Table 3).
Sprainting activity varied significantly with season, river characteristics, elevation and disturbance. The logistic regression model included variables relating to habitat highlighting that the physical characteristics of the river and surrounding vegetation cover were critical to the presence or absence of otter sprainting at different survey seasons.
Spraints occurred more often than expected where rocky and hard sand was found in the immediate vicinity (15 x 100 m strip width plot) of the survey site, and where other covariates were found in the surrounding land (100 x 15 m strip-width cell neighborhood). Spraints were found less often than expected at sites where dry leaf litter cover was found either in the surrounding land or the immediate vicinity, and low canopy cover (Table 4).
A significant χ2 value indicates that there was a significant association between the presence or absence of spraints and that habitat type. A positive association of sprainting with particular habitat types are indicated by a plus sign and a negative association by a minus sign in the table.
Factors influencing Asian Small-Clawed Otter Occurrence in Post-Monsoon Winter and Summer Seasons in the Moyar River
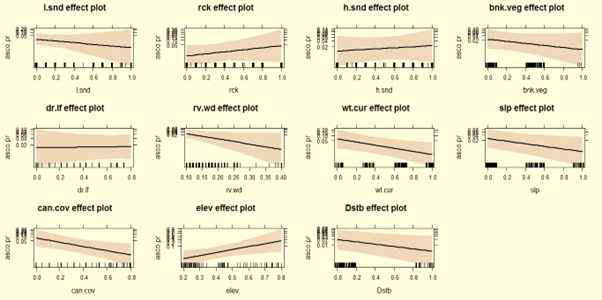
The logistic regression model explained 80.23% of the variance in otter habitat use. Post-monsoon factors were positively related with altitude/elevation (z=2.347, p<.01), rocky stretches (z=1.043, P<.05), hard sand (z=0.492, P<0.05), disturbance (z=2.025, P<0.05) but negative related with water current, (z=-2.363, P<0.01), river width (z=-1.973, P<0.01), and canopy cover (z=-2.363, P<0.01). During winter, otter occurrence was positively related with altitude/elevation (z=4.129, P<00.01), disturbance (z=1.708, P<0.01), and negative related with river width (z=-3.155, P<0.001), water current (z=1.946, P<0.01) and hard sand (z=-2.441, P<0.01). In summer, otter occurrence was positively related with altitude/elevation (z=2.347, P<0.01), and negatively correlated with water current (z= -2.362, P<0.01), river width (z=-2.181 P<0.01), canopy cover (z=-2.362, P<0.01) and hard sand (z=-2.441, P<0.01; Table 5).
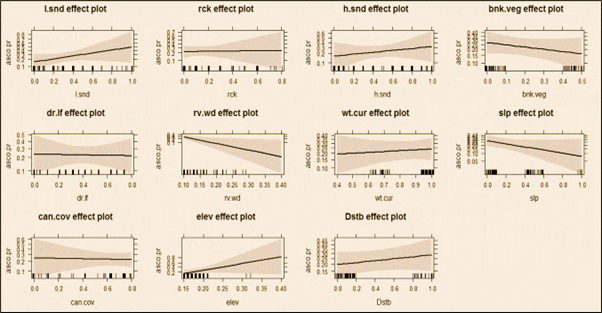
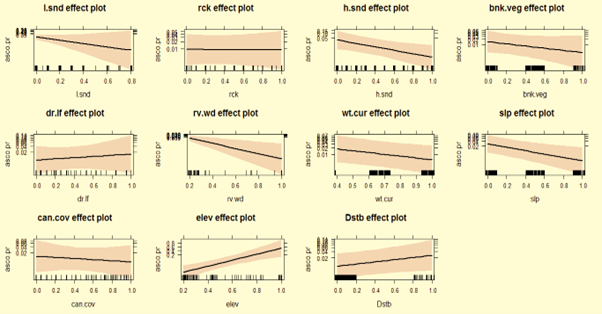
After the monsoon, Asian small-clawed otters preferred rocky areas with high altitude/elevation, rocky stretches, stagnant water current, and avoid wider river, medium water flow, and dense canopy cover (Fig. 2). In winter, otters maintained their preferences for rocky areas with high altitude/elevation, but avoided wider, deeper river sites in lower altitude and stagnant water flow (Fig. 3). In summer, Asian small-clawed otters preferred higher altitude/elevation, shallow water, rocky areas with boulders, and a moderate canopy, while avoiding anthropogenic disturbance, loose and hard sandy areas, stagnant water flow, and lower elevations similar to previous seasons (Fig. 4). Otter sign occurrence varied with water current (i.e., fast, slow and stagnant), shallow water plots yielded more spraints. No spraints were recorded at plots (129 plots) where wattle had invaded > 89% shore vegetation cover. Spraints were most frequently recorded at plots with moderate shoreline vegetation.
DISCUSSION
Occurrence-based surveys are particularly useful for conservation assessments of poorly studied and elusive species, such as the Asian small-clawed otter. This approach can be used even in single-time surveys to generate rigorous baseline data when detectability is adequately accounted (Beja, 1992). At the same time, it also allows ecological questions on the determinants of finer scale habitat selection to be addressed (Perinchery et al., 2011). Policy-based action, research on factors affecting survival, habitat-based actions on creation and, where required, expansion of protected areas and communication and awareness building among local communities are suggested by Hussain (1999) as fundamental to the conservation of the Asian small-clawed otter. This is first ever attempted to address the factors affecting the resource use of Asian small-clawed otter from this region. Ultimately, altitude/elevation and river characteristics played an important role in determining the presence of Asian small-clawed otter. This is consistent with natural history accounts of the species in southern India (Pocock, 1941; Perinchery et al., 2011) and highlights the importance of high-altitude habitats for the conservation of this species (> 660 m asl) . In Southeast Asia, however, the species also occurs in low-elevation habitats, such as wetland systems with pools and stagnant water (Hussain,1998; Sivasothi and Nor, 1994). Further detailed surveys would be necessary across a greater altitudinal gradient across southern Western Ghats to determine if the distribution of the species also includes lower-elevation regions (e.g., below the <650 m asl of this study).
Having selected home ranges based on altitude for a given number of seasons, otters seem to concentrate habitat use at higher altitudes within their occupied streams. The preference for high altitude river stretches over cascades and riffles is unsurprising given that the species is a specialized feeder on crustaceans and molluscs, locating prey mainly by touch (Ewer 1973). Elevation gradient, river substrate, grass cover, and ground cover along the banks also was a better predictor of otter habitat use than compared to stream order (Arden-Clarke, 1986; Perinchery et al., 2011). Our study demonstrates the utility of modeling occurrence-based detectability simultaneously (Mackenzie et al., 2002). Several recent studies have successfully applied the approach to estimate sign detectability, and the role of habitat-related and other covariates in determining these, for a range of species (e.g., Baldwin and Bender, 2008; Bonesi and Macdonald, 2004; Buckley and Beebee, 2004; Carter et al., 2006; Martin et al., 2006; Mazerolle et al., 2005; Moritz et al., 2008; O’Connell et al., 2006; Welsh et al., 2008).
The identification of the species’ preference for higher altitudes and pits/pools in a protected area will help identify sites for conservation efforts targeting Asian short-clawed otters; high-altitude streams and small pools should be seen as important conservation zones in unprotected areas as well, because they can be prospective habitats for this species, if it does not currently occupy the area. This study also can help direct future surveys by predicting the presence of Asian small-clawed otter in similar areas. Given that this study was conducted in the partially disturbed Moyar River, the findings indicate patterns of habitat use in the presence of human disturbance or habitat modification. Therefore, Asian small-clawed otter required fine-scale environmental factors in each season to satisfy their habitat requirements, and adaptive management to conserve those factors is needed for their long-term survival in a human-dominated landscape, such as the Moyar. However, the majority of freshwater habitats in southern India are not protected and are located within or near human-dominated areas (Perinchery et al., 2011), and anthropogenic impacts on riparian landscapes can entirely alter species composition (Jelil et al., 2021). Otters tend to avoid human presence either spatially or temporally by restricting activity to certain seasons (Rosas et al., 2007; Shenoy et al., 2006; Tuzun and Albayrak, 2005). But, in Moyar they tend to tolerate human presence up to certain level during post monsoon and winter seasons (Figure 2, 3).
CONCLUSION
Although our study has developed detailed data on factors that are influence the species’ persistence in protected habitats, future studies also should focus on anthropogenic changes in otter habitats and the consequences for survival of Asian small-clawed otter populations. We recommend a comprehensive survey covering the entire distribution of the species in the Western Ghats of southern India, spanning wide elevational, latitudinal, altitudinal, habitat and disturbance-related gradients to obtain an understanding of regional status and threats faced by Aonyx cinereus nirnai.
Acknowledgments - We thank our respective institution for supporting our research activities. Special thanks to Tamil Nadu Forests Department for providing necessary permission. Field Directors (Sathyamangalam & Mudumalai Tiger Reserves), District Forest Officer (Nilgiri North Division), and the Forest Range Officers are thanked for kindly supporting the fieldwork. We are deeply grateful to our forest warriors (anti-poaching watchers) for their help during the field work. KN sincerely thanks to Mr. Abhishek Gopal for his undisputable help during the fieldwork. We thank our anonymous referees for their constructive comments, which helped us to improve the quality of the manuscript.
Author Contributions: KN conceived & designed the study, data analysis and drafted the manuscript; KN and SP performed the data collection; MWH & MTM helped draft the final manuscript.
Research funding: KN received funding assistance from “Conservation Leadership Programme (ID: 03228615)” “Rufford Foundation (ID: 17667-1)” and “Idea Wild (ID: NARAINDIA0415)”.
Conflict of interest statement: The authors declare that they have no conflicts of interest regarding this article.
Research Ethics/Best Practice: This research was conducted in the protected areas with proper permission from the Tamil Nadu Forests department, which was given to KN and his team via permit number WL5/20861/2015 and Ref. No. 6612/2015M. We adopted a non-invasive technique (field survey and camera trapping) to collect the data and no animals were harmed or handled during this study.
REFERENCES
Anoop, K.R. and Hussain, S.A. (2004). Factors affecting habitat selection by Smooth-coated otters (Lutra perspicillata) in Kerala, India. Journal of Zoology. 263: 417-423. https://doi.org/10.1017/S0952836904005461
Arden-Clarke, C.H.G. (1986). Population density, home range and spatial organization of the cape clawless otter (Aonyx capensis) in a marine habitat. Journal of Zoology. 209: 201-211. https://doi.org/10.1111/j.1469-7998.1986.tb03576.x
Baldwin, R.A. and Bender, L.C. (2008). Distribution, occupancy and habitat correlates of American martens (Martes americana) in Rocky Mountain National Park, Colorado. Journal of Mammalogy. 89: 419–427. https://doi.org/10.1644/07-MAMM-A-053R1.1
Beja, P.R. (1992). Effects of freshwater availability on the summer distribution of otters (Lutra lutra) on the southwest coast of Portugal. Ecography. 15: 273-278. https://doi.org/10.1111/j.1600-0587.1992.tb00035.x
Bonesi, L. and Macdonald, D.W. (2004). Evaluation of sign surveys as a way to estimate the relative abundance of American mink (Mustela vison). Journal of Zoology. 262: 65-72. https://doi.org/10.1017/S0952836903004448
Brzeziński, M. and Jedrzejewska, B. (1993). Diet of otters (Lutra lutra) inhabiting small rivers in the Bialowieza National Park, Eastern Poland. Journal of Zoology. 230: 495-501. https://doi.org/10.1111/j.1469-7998.1993.tb02701.x
Buckley, J. and Beebee. T.J.C. (2004). Monitoring the conservation status of an endangered amphibian: the natterjack toad Bufo calamita in Britain. Animal Conservation. 7: 221–228. https://doi.org/10.1017/S1367943004001428
Carter, G.M., Stolen, E.D., and Breininger. D.R. (2006). A rapid approach to modeling species–habitat relationships. Biological Conservation. 127: 237–244. https://doi.org/10.1016/j.biocon.2005.08.012
Champion, H.G. and Seth, S.K. (1962). A revised survey of the forest types of India. Manager of Publications. Delhi. 404pp. https://archive.org/details/foresttypesofind0000sirh/page/n3/mode/2up
Ewer, R.F. (1973). The Carnivores. Cornell University Press, Ithaca, New York. ISBN: 978-0801407451
Guter, A., Dolev, A., Saltz, D., & Kronfeld-Schor, N. (2008). Using videotaping to validate the use of spraints as an index of Eurasian otter (Lutra lutra) activity. Ecological indicators, 8(5): 462-465. https://doi.org/10.1016/j.ecolind.2007.04.009
Hussain, S.A. (1998). Conservation status of otters in the Terai and Lower Himalayas of Uttar Pradesh, India. pp. 1-13. In: Proceedings of the VII International Otter Symposium, March 13-19, 1998, Trebon, Czech Republic. https://www.iucnosgbull.org/Volume19A/Trebon-II.pdf
Hussain, S.A. (1999). Otter conservation in India. Envis Bulletin - Wildlife and Protected Areas. 2 (2): 92-97.
Hussain, S.A. and Choudhury, B.C. (1995). Seasonal movement, home range and habitat use by Smooth-coated otters Lutra perspicillata in National Chambal Sanctuary, India. pp. 45-55. In: Reuther, C., Rowe-Rowe, D. (Eds.). Proceedings of VI. International Otter Colloquium, Pietermaritzburg. Aktion Fischofterschutz Hankensbüttel.
Hussain, S.A., de Silva, P.K. (2008). Aonyx cinerea. IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/44166
Jelil, S.N., Gaykar, A., Girkar, N., Ben, C., Hayward, M.W., and Krishnamurthy, R. (2021). Mammal Persistence Along Riparian Forests in Western India Within a Hydropower Reservoir 55 Years Post Construction. Front. Ecol. Evol. 9:643285. https://doi.org/10.3389/fevo.2021.643285 .
Kruuk, H. (1995). Wild otters - Predation and populations. Oxford University Press, 287pp. ISBN: 9780198540700
Macdonald, S.M. and Mason, C.F. (1983). Some factors influencing the distribution of otters (Lutra lutra). Mammal Review,13: 1-10. https://doi.org/10.1111/j.1365-2907.1983.tb00259.x
Mackenzie, D.I. (2006). Modeling the probability of resource use: the effect of, and dealing with, detecting a species imperfectly. Journal of Wildlife Management. 70: 367–374. https://doi.org/10.2193/0022-541X(2006)70[367:MTPORU]2.0.CO;2
MacKenzie, D.I., Nichols, J.D., Lachman, G.B., Droege, S., Andrew Royle, J., and Langtimm, C.A. (2002). Estimating plot occurrence rates when detection probabilities are less than one. Ecology. 83: 2248-2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
Martin, T.G., Mcintyre, S., Catterall, C.P., and Possingham, H.P. (2006). Is landscape context important for riparian conservation? Birds in grassy woodland. Biological Conservation. 127: 201–214. https://doi.org/10.1016/j.biocon.2005.08.014
Mazerolle, M.J., Desrochers, A., and Rochefort, L. (2005). Landscape characteristics influence pond occupancy by frogs after accounting for detectability. Ecological Applications. 15: 824–834. https://doi.org/10.1890/04-0502
Medina, G. (1996). Conservation and status of Lutra provocax in Chile. Pacific Conservation Biology, 2: 414-419. https://doi.org/10.1071/PC960414
Meena, V. (2002). Otter poaching in Palni Hills. Zoo’s Print Journal. 17 (2): 696-698. https://zoosprint.org/index.php/zpj/article/view/5977
Melquist, W.E. and Hornocker, M.G. (1983). Ecology of river otters in west central Idaho. Wildlife Monographs 83:1-60. https://www.jstor.org/stable/3830731
Mohapatra, P.P., Palei, H.S., and Hussain, S.A. (2014). Occurrence of Asian small-clawed otter Aonyx cinereus (Illiger, 1815) in Eastern India. Current Science, 107 (3): 367-370. https://www.researchgate.net/publication/263366590
Moritz, C., Patton, J.L. Conroy, C.J., Parra, J.L., White, G.C., and Beissinger, S.R. (2008). Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 322: 261–264. https://doi.org/10.1126/science.1163428
Narasimmarajan, K. (2020). Ecology of otters (Carnivore, Mustelidae) in the Moyar River, Western Ghats, Southern India. Unpublished PhD Thesis. The University of Madras. 235pp.
Narasimmarajan, K., Gopal, A., Palanivel, S., and Mathai, M.T. (2018). Status of Mugger Crocodiles (Crocodylus palustris) in River Moyar, Southern India. Cobra. XII (2): 1- 9. https://ruffordorg.s3.amazonaws.com/media/project_reports/Cobra%2C%20Vol.%20XII%2C%20Issue%202.%202018.PDF
Narasimmarajan, K., Hayward, M.W., and Mathai, M.T. (2021). Assessing the Occurrence and Resource Use Pattern of Smooth-Coated Otters Lutrogale perspicillata Geoffroy, 1826 (Carnivore, Mustelidae) in the Moyar River of the Western Ghats Biodiversity Hotspot. IUCN Otter Spec. Group Bull. 38: (1): 43–58. https://www.iucnosgbull.org/Volume38/Narasimmarajan_et_al_2021.html
Narasimmarajan, K., Hayward, MW. Palanivel, S. and Mathai, MT. (2023). Population status and temporal activity pattern of two Vulnerable otter species from camera-trapping in the Southern Western Ghats biodiversity hotspot. IUCN Otter Spec. Group Bull. 40 (4): 183 – 196. https://www.iucnosgbull.org/Volume40/Narasimmarajan_et_al_2003.html
Narasimmarajan, K., Palei, H.S., and Mathai, M.T. (2024). Do Natural River Pits pose a Danger to Otters? A Field Report from the Moyar River, Western Ghats, India. IUCN Otter Spec. Group Bull. 41 (1): 24 – 30. https://www.iucnosgbull.org/Volume41/Narasimmarajan_et_al_2024.html
Nawab, A. and Hussain, S.A. (2012). Factors affecting the occurrence of smooth-coated otter in aquatic systems of the Upper Gangetic Plains, India: Smooth-Coated Otter in Upper Gangetic Upper Plains. Aquatic Conservation: Marine and Freshwater Ecosystem. 22: 616-625. https://doi.org/10.1002/aqc.2253
Newman, D.G.and Griffin, R. (1994). Wetland use by river otters in Massachusetts. Journal of Wildlife Management. 58: 18-23. https://doi.org/10.2307/3809544
O’Connell, A.F., Talancy, J.R., Bailey, N.L.L., Sauer, J., Cook, R., and Gilbert, A.T. (2006). Estimating site occupancy and detection probability parameters for mammals in a coastal ecosystem. Journal of Wildlife Management. 70: 1625–1633. https://doi.org/10.2193/0022-541X(2006)70[1625:ESOADP]2.0.CO;2
Palei, H.S., Mohapatra, P.P., and Hussain, S.A. (2023). Habitat selection and diet of the Asian small-clawed otter in Karlapat Wildlife Sanctuary, Odisha, India. Écoscience. 30: (1) 17-26. https://doi.org/10.1080/11956860.2023.2165020
Perinchery, A., Jathanna, D., and Kumar, A. (2011). Factors determining occupancy and habitat use by Asian small-clawed otters in the Western Ghats, India. Journal of Mammalogy. 92:(4) 796–802. https://doi.org/10.1644/10-MAMM-A-323.1
Pocock, R.I. (1941). The fauna of British India including Ceylon and Burma. Mammalia. Vol. 2. Carnivora. Taylor and Francis, London, United Kingdom. https://archive.org/details/PocockMammalia2/mode/2up
Prakash, N., Mudappa, D., Raman, T.S.R., and Kumar, A. (2012). Conservation of the Asian Small-Clawed Otter (Aonyx Cinereus) in Human-Modified Landscapes, Western Ghats, India. Tropical Conservation Science. 5 (1): 67-78. https://doi.org/10.1177/194008291200500107
Puyravaud, J.P. and Davidar, P. (2013). The Nilgiris Biosphere Reserve: an unrealized vision for conservation. Tropical Conservation Science. 6: 468-476. https://doi.org/10.1177/194008291300600401
R Core Team. (2018). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/.
Raha, A. and Hussain, S.A. (2016). Factors affecting habitat selection by three sympatric otter species in the southern Western Ghats, India. Acta Ecologica Sinica 36: 45–49 https://doi.org/10.1016/j.chnaes.2015.12.002
Rosas, f.C.W., Demattos, G.E., and Cabral, M.M. (2007). The use of hydroelectric lakes by giant otters Pteronura brasiliensis: Balbina Lake in central Amazonia, Brazil. Oryx. 41: 520–524. https://doi.org/10.1017/S0030605307005121
Shenoy, K., Varma, S., and Devi Prasad, K.V. (2006). Factors determining habitat choice of the smooth-coated otter, Lutra perspicillata in a South Indian River system. Current Science. 91: 637–643. https://www.jstor.org/stable/24094370
Sivasothi, N. and Nor, B.H.M. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia. 285: 151–170. https://doi.org/10.1007/BF00005663
Tuzun, I. and Albayrak, I. (2005). The effect of disturbances to habitat quality on otter (Lutra lutra) sprainting activity in the River Kizilirmak (Turkey): a case study. Turkish Journal of Zoology. 29: 327–335. https://journals.tubitak.gov.tr/zoology/vol29/iss4/7
Van Emden, H.F. (2008). Statistics for terrified biologists. Blackwell Pub. ISBN: 978-1405149563
Welsh, H.H., Pope, J.R., and Wheeler, C.A. (2008). Using multiple metrics to assess the effects of forest succession on population status: a comparative study of two terrestrial salamanders in the US Pacific Northwest. Biological Conservation. 141: 1149–1160. https://doi.org/10.1016/j.biocon.2008.02.014
White, P.C.L. McCleana, C.J., and Woodroffe, G.L. (2003). Factors affecting the success of an otter (Lutra lutra) reinforcement programme, as identified by post-translocation monitoring. Biological Conservation. 112: 363-371. https://doi.org/10.1016/S0006-3207(02)00333-6
Wright, L., de Silva, P.K., Chan, B., Reza Lubis, I. and Basak, S. (2021). Aonyx cinereus. The IUCN Red List of Threatened Species 2021: e.T44166A164580923. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T44166A164580923.en.
Zar, J.H. (1984). Biostatistical analysis. Ind. edn. Prentice-Hall Inc., New Jersey. 718pp. ISBN: 9780130815422
Résumé: Des Mustelidés Méconnus : Rôle des Variables Environnementales dans l’Influence des Modèles Saisonniers d’Utilisation des Ressources Des Loutres Cendrées (Aonyx cinereus nirnai Illiger, 1815) dans la Rivière Moyar du Point Chaud de la Biodiversité des Ghats Occidentaux, en Inde
Nous avons examiné le rôle des variables environnementales influençant les schémas d’utilisation des ressources des loutres cendrées (Aonyx cinereus nirnai) en échantillonnant l’ensemble de la rivière Moyar des Ghâts occidentaux entre mars 2015 et septembre 2017, en utilisant les indices de présence de loutre comme indicateur. Un cadre basé sur l’occurrence a été utilisé pour déterminer l’influence des covariables environnementales sur la détectabilité des loutres. Les informations sur les paramètres environnementaux ont été enregistrées chaque fois que des indices de présence de loutre ont été détectés ou non détectés sur des sites espacés de 400 mètres le long des berges de la rivière pendant les saisons post-mousson, hivernale et estivale. La détectabilité des indices de présence de loutre était influencée par le substrat de la rivière, les caractéristiques de l’habitat, les caractéristiques des berges et les types de forêts. Les loutres préfèrent la haute altitude/élévation, les rivières étroites et les zones rocheuses aux eaux peu profondes, mais évitent les zones de rivières sablonneuses, plus larges et profondes. Les schémas d’utilisation des ressources ont été déterminés par les caractéristiques de la rivière et de l’habitat au cours des trois saisons. Diverses formes de perturbation ont eu un effet négatif sur la présence des loutres. Les loutres cendrées ont besoin de caractéristiques environnementales spécialisées et spécifiques à leur habitat pour survivre à long terme dans un paysage dominé par l’homme. La restauration des habitats dégradés et des sites envahis par des acacias non indigènes est essentiel pour améliorer les perspectives de conservation à long terme de la loutre cendrée. Les plans de conservation des loutres doivent être spécifiques à chaque espèce pour aider à maintenir l’équilibre écologique de l’écosystème de la rivière Moyar.
Revenez au dessus
Resumen: Centinelas Menos Conocidos: Influencia de las Variables Ambientales en los Patrones Estacionales de Uso de Recursos por las Nutrias de Uñas Pequeñas Asiáticas (Aonyx cinereus nirnai Illiger, 1815) en el Río Moyar, Hotspot de Biodiversidad de los Ghats Occidentales, India
Examinamos el rol de variables ambientales que influencian los patrones de uso de recursos por las nutrias de uñas pequeñas Asiáticas (Aonys cinereus nirnai), muestreando la totalidad del Río Moyar, Ghats Occidentales, entre marzo de 2015 y Septiembre de 2017, utilizando los signos de nutria como indicador. Utilizamos un marco basado en la ocurrencia para determinar la influencia de las covariables ambientales en la detectabilidad de las nutrias. Registramos información sobrre parámetros ambientales cada vez que se detectaban o no se detectaban signos de nutria en sitios espaciados cada 400 metros a lo largo de las barrancas del río, durante las estaciones post monzones, invierno, y verano. La detectabilidad de los signos de nutria estuvo influenciada por el sustrato del río, las características del hábitat, los rasgos de la barranca del río, y los tipos forestales. Las nutrias prefieren ríos en alta altitud/elevación, angostos, y áreas rocosas con aguas poco profundas, pero evitaron áreas del río arenosas, más anchas y más profundas. Los patrones de uso de recursos estuvieron determinados por las características del río y del hábitat, en las tres estaciones. Varias formas de disturbio afectaron en forma adversa la ocurrencia de las nutrias. Las nutrias de uñas pequeñas Asiáticas requirieron rasgos ambientales especializados, específicos de hábitat, para prosperar a largo plazo en éste paisaje dominado por el ser humano. Es necesaria la restauración de los hábitats degradados y de los sitios invadidos por el zarzo dorado (no nativo), para mejorar las perspectivas de conservación a largo plazo de la nutria de uñas pequeñas Asiática. Los planes de conservación de nutrias deben ser especie-específicos para mantener el balance ecológico en el ecosistema del Río Moyar.
Vuelva a la tapa