IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 36(A) Special Issue (April 2019)
 PROCEEDINGS OF THE 14th INTERNATIONAL OTTER CONGRESS
PROCEEDINGS OF THE 14th INTERNATIONAL OTTER CONGRESS
8 - 19th April 2019
Tangjiahe, China
Relationship between Microhabitat Structure and Otter Presence in an Oil Palm Dominated Landscape of Sabah, Malaysia
Annabel Pianzin1 2 *, Anna Wong1 2 , and Henry Bernard1
Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia.
2Malaysian Nature Society, JKR 641 Jalan Kelantan, Bukit Persekutuan, 50480 Kuala Lumpur, Federal Territory of Kuala Lumpur.
* Corresponding Author criikey.mate@yahoo.com
Received 3rd July 2019, accepted 2nd March 2020
Abstract: Land-use changes derived from agricultural expansion and urbanization is a global conservation concern in Southeast Asia. Impacts from these activities particularly oil palm plantation intensification has a detrimental effect on aquatic habitats, leading to biodiversity loss and degraded water quality. Riparian species such as otters whose habitats expand linearly beyond undisturbed habitats may be sensitive towards human-modified landscapes due to changes in the surrounding forests and water bodies. The present study investigates the relationship between microhabitat structure and otter presence based on tracks and spraints in an oil palm dominated landscapes located in southeastern Sabah, Malaysian Borneo. To examine the relation between habitat parameters and otter occurrence, we conducted Principle Component Analysis (PCA) and Generalized Linear Model (GLM). Several microhabitat structures were found to be positively associated with otter presence. Results from the GLM analysis showed that substrates with high exposed soil or a combination of exposed soil and rocks, and substrate with low rock content supported higher otter presence. The proximity to oil palm plantations is also a good predictor of otter presence with a positive effect, where streams located closer to plantations contained higher signs of otters. In contrast, the presence of otter was negatively affected by narrow stream width, narrow stream banks and stream located further away from the nearest human settlement. Result of this study revealed the persistence of otters in human-modified areas especially in oil palm dominated landscapes provided that important habitat parameters are present for otter activities (sprainting, grooming, denning) and for successful conservation planning.Keywords: oil palm, land-use changes, human-modified landscapes, otter, microhabitat structure
INTRODUCTION
Modification of natural forests into agricultural plantations is the major driver of biodiversity loss, especially oil palm in Southeast Asia (Fisher et al., 2011; Koh and Wilcove, 2008; Sodhi et al., 2004; Tilman et al., 2001). These changes are known to negatively affect freshwater ecosystem because of the structure of riparian zones and distinct vegetation community affecting every level of the trophic network (Feld et al., 2011; Thomas et al., 1979). Riparian species such as otters are directly influenced by agricultural activities when important habitat features i.e. spatially complex riparian forests converted into a much simpler vegetation structure (Danielsen et al., 2009; Foster et al., 2011) and their prey base declined by degraded stream quality (Bedford, 2009; Struebig et al., 2008). As an adaptation towards decreasing undisturbed habitats, otters are forced to move into modified forests as foraging, breeding or refuge grounds (Bhagwat et al., 2008; Gardner et al., 2009; Laws, 2006). Due to the linear nature of otter habitat, they are more sensitive towards the impacts of human-modified landscapes (Prakash et al., 2012). In addition, the threats affecting riparian carnivores in such habitat is poorly understood (Laws, 2016) and there is a lack of knowledge on habitat requirements of semi-aquatic vertebrates in an oil palm dominated landscapes. The aim of this study was to identify important microhabitat structure that influence the presence of the common otter species in Sabah, the Smooth-coated Otter (Lutrogale perspicillata) and the Asian Small-clawed Otter (Aonyx cinereus), in an area dominated by oil palm plantations where these plantations (established between 1998 and 2012) are situated neighboring twice-logged lowland dipterocarp rainforest and acacia plantations (Gray et al., 2015; Mitchell et al., 2018).
METHODOLOGY
Study Area
The present study was conducted in and around the ‘Stability of Altered Forest Ecosystems’ (SAFE) Project (117.5oN, 4.6oE) area located within the Kalabakan Forest Reserve, in southeastern Sabah, Malaysian Borneo (Figure 1). The area consists of around 80,000 ha of twice-logged dipterocarp rainforest, acacia and oil palm plantation planted between 1998 and 2012. SAFE Project has been gazetted for the conversion of plantation in the last 20 years where heavily altered logged forests is converted into oil palm plantations with a network of riparian reserves and fragmented forests retained for scientific research, see Ewers et al. (2011) for more information. It has high annual rainfall of up to 2925 mm with monthly mean of 450 mm and little climate seasonality (where drought takes place only in major El Niño Southern Oscillation years) (Luke et al., 2017; Malaysian Meteorological Department, 2018).
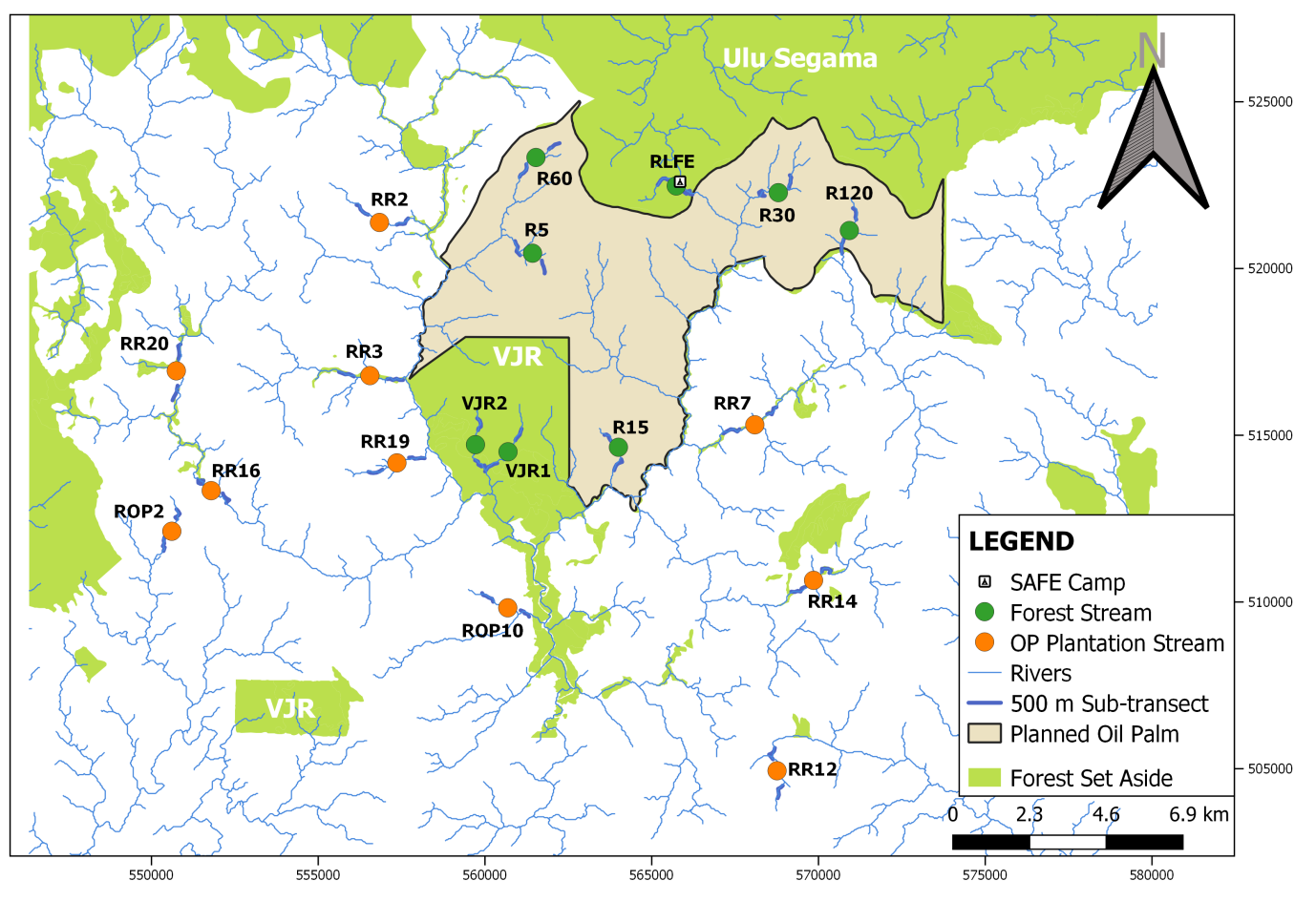
A total of 18 riparian sites were selected within the study area (Figure 1, Table 1 ), where 8 streams were located within forests (continuous and fragmented) and 10 streams located within oil palm plantations (with or without riparian buffer strips). The selected streams were located in areas of different disturbance and alteration level since they were the standard of main habitat changes found in this region (Reynolds et al., 2011). Data collection was conducted before the conversion into oil palm plantation within the experimental area, which will provide useful data for comparison between pre and post-conversion.
| Table 1: Location of surveyed streams in forested areas and agricultural sites | |||
| Habitat Type | Habitat Treatment | Stream | Habitat Description |
| Forested Area | Old Growth Forest (Continuous) |
VJR1 VJR2 |
Located in a 2,200 ha virgin jungle reserve hich were lightly logged around the edges in 1970s and 1990s for road construction but not to the extent of commercial selective logging. Old growth forest features remain intact. |
| Logged Forest (Continuous) | RLFE | Located in twice-logged forest adjoining the Ulu Segama Forest Reserve, encompassing three large conservation areas (Danum Valley, Maliau Basin and Imbak Canyon) where all three have never been logged (greater than 1 million ha). It was selectively harvested in 1970s and late 1990s to early 2000s, although 71% of its forest cover remains intact | |
| Heavily Degraded Forest (Fragmented)0 |
R5,
R15, R30, R60, R120 |
Located in fragmented forest with varying buffer width from 5 up to 120 m, consists of 7,200 ha of lowland dipterocarp rainforest. Undergone multiple rounds of selective logging (two to four times) in the 1970s, from late 1990s to 2000s and recent disturbance in 2015. Holds a small percentage of mature trees, although some areas are less disrupted and are protected by law. Highly heterogeneous with forest patches of closed canopy intermixed with regrowth, gaps and roads. Majority had been designated for the conversion to oil palm plantation by the Malaysian government. | |
| Agricultural Site | Riparian Reserve within Plantation (Buffer Zone) |
RR2,
RR3, RR7, RR12, RR14 RR16 RR19, RR20 |
Riparian buffer strip retained in mature oil palm plantation planted between 1999 and 2009. The reserves each have difference in size ranging from 20 – 120 m in width. They vary in topography, altitude, stream, and bank substrate as well as ruggedness. |
| Oil Palm Plantation (No Buffer Zone) |
ROP2, ROP10 |
Located in the adjoining oil palm plantation located immediately outside the experimental area with no buffer strips. | |
| Source: Ewers et al. (2011); Laws (2016); Luke et al. (2017); Mitchell et al. (2018); Struebig et al. (2013). | |||
Data Collection
We examined the correlation between microhabitat structure in an oil palm dominated landscape with the occurrence of otters in a Principle Component Analysis (PCA) and Generalized Linear Model (GLM) framework, using the absence and presence of otters as an indicator.
The field survey was carried out between January 2017 to June 2018 where visual signs survey was conducted along stream banks across 18 streams. Each survey was conducted along a 2.0 km line transect where a stream consisted of two 500 m non-overlapping sub-transect separated by a 1.0 km gap avoid repeated counting of the same individual in multiple segments (Laws, 2016). Each stream was surveyed on four occasions to confirm the presence or absence of otters. Site visitation and replication were randomized across streams surveyed with at least two days to three months apart. The survey was conducted on foot and otter signs were thoroughly searched on both sides of the stream banks as well as rocks and boulders in the streams. At least two trained observers searched for otter signs, one at each bank. The overall sampling effort over the extent of 72 days survey occasions was about 205 hours with distance travelled within the 2 km line transect across 18 streams at 144 km.
Identification of otter signs were based on the overall appearance and measurement of tracks, and appearance and prey content of spraints. Spraints were aged (≥ 2 days, < 2 days) to prevent repeated samplings although signs were always fresh due to regular rainfall. Undigested prey remains of all spraints were documented and identified based on the presence of fishes (bones and scales), crustaceans, other invertebrates, small mammals, and other prey items with regards to spraint appearance. Tracks were measured by width and length, and were classified according to species by the presence of claw marks and type of webbing (partially or fully webbed). Photographs of signs found were taken with a digital camera and coordinates of each sign were recorded using a GPS handheld.
Habitat characteristics were determined by stream and stream bank structures recorded at regular interval along 100 m segment within the 500 m sub-transect. Habitat characteristics were taken to primarily observe the relationship between otter and difference in habitat structures as these habitat parameters may influence their presence. Vegetation plots of 5 x 5 m dimension were applied at each side of the banks to verify bankside vegetation condition, both at canopy and ground level which are important environment features for otters. Fifteen stream and stream bank habitat covariates were measured. Refer to Table 2 for the list of recorded habitat variables with its corresponding method of measurement.
Data Analysis
Bank substrates were converted into proportion in sub-transects as exposed soil/sand, rocks or a combination of both. The proportioned data were then transformed using ‘arcsine’ transformation. All subsequent analyses were conducted using the program RStudio version 1.1.463 (R Core Team, 2019). Habitat variables were transformed and normalized to have a mean of zero and variance of one to help in limiting the influence of dimensions in each variables and different scales used (Mackenzie et al., 2006; Muanis and Oliveira, 2011). Analysis was carried out by including habitat parameters of all habitat types to get an overall representation across the landscape level due to the linear nature of otter’s habitat.
Principle Component Analysis (PCA) was conducted using the package “prcomp” to reduce a large set of variables into a smaller set but still includes most of the information from the large dataset and limits collinearity (predictor variables that are highly correlated among temselves) in the recorded habitat variables. Principle components (PC) axes with eigenvalues of > 1 were selected to be inputted into Generalized Linear Model (GLM) using the package “brglm” to examine relationship between the 15 habitat variables of 36 stream segments with regards to presence of otters in oil palm dominated landscape. Binomial error family and logit link function were used in the GLM formula. Otter occurrence was documented as ‘1’ for presence and ‘0’ for absence.
RESULT
Visual signs survey revealed the presence of two otter species in the study area: Small-clawed Otter (A. cinereus) and Smooth-coated Otter (L. perspicillata). A total of 82 otter signs were detected where 36 signs belonged to L. perspicillata (42.86%) and 48 signs belonged to A. cinereus (57.14%). Out of the 36 sub-transect surveyed, 24 (66.67%) proved positive for otter presence.
Microhabitat Structure
The 17 recorded habitat variables were reduced to four principle components (PC) where it explains 70.0% of the variation in the system (Table 3). The first principle component (PC1) contained stream canopy cover, undergrowth cover, bank canopy cover, forest quality, and bank tree height (32.8%). The second component (PC2) contained type of bank substrate (proportions of rock, soil/sand or combination of both), and distance to oil palm estate (17.00%). The third component (PC3) contained stream width, stream edge, and distance to human settlement (11.0%) and lastly, the fourth component (PC4) contained stream depth, number of fallen logs, and bank tree DBH (9.2%).
The second component was positively correlated to otter presence where streams contained banks with high exposed soil or a combination of exposed soil and rock, less exposed rocks, and located in close proximity to oil palm estates (Z=0.0152, P=0.0152). Conversely, the third component was negatively correlated to otter presence where streams possess narrow width, narrow banks, and located far from the nearest human settlement (Z=-1.963, P=0.0497). Streams with well covered bank canopy, good forest quality, and trees with small trunk circumference (Z=1.266, P=0.2057), as well as deep streams, many fallen logs and bank trees with small trunk circumference (Z=-0.409, P=0.6824) indicate no significant correlation to the presence of otter.
DISCUSSION
The GLM analysis identified several microhabitat structures that was correlated to the presence of otter signs in an oil palm dominated landscape which included stream width, distance of stream edge, proportion of bank substrates, and distance of stream to oil palm estates and human settlements.
The presence of otters increased when proportion of exposed soil or a combination of both soil and rocks increased. In contrast, otter presence decreased as proportion of exposed rocks increased. According to several studies (Anoop and Hussain, 2004; Hon et al, 2010; Kruuk, 2006; Prakash et al., 2014; Mason and Macdonald, 1987), otters have a high preference to substrate with higher soil content (mainly sand) as it is associated with grooming due to its dry nature which absorbs moisture faster, a vital feature of their habitat. Rocks are important for depositing spraints, shelter, and habitat for prey items (Hussain, 1993; Hussain and Choudhury, 1997). Soil substrate (mainly sandy banks) makes it easier to identify otter tracks, whereas tracks are difficult to identify on gravels and footprints do not imprint on larger rocks sizes. However, sighting of spraints was easily observed on rocks and boulders. The findings may be biased to the type of substrate with the type of otter signs observed. Most of the signs included in the analysis were tracks and was detected on sandy substrate. Whereas spraints were strongly tied to rocky substrate and were mostly detected outside of the sub-transects and thus excluded from the analysis.
The close proximity of streams to oil palm estates showed a positive effect on otter presence. They are known to be tolerant towards human disturbance, but are usually crepuscular or nocturnal in areas where humans are present (Kanchanasaka and Duplaix, 2011). High occurrence of otters in human-modified landscapes connecting protected areas shows they are rather unreceptive to this habitat due to the linear nature of otter habitat, allowing them to move further into disturbed environment (Cho et al., 2009; Foster-Turley, 1992; Prakash et al., 2012; Sepúlveda et al., 2007). Since there is a limited high quality habitat in the study area, both A. cinereus and L. perspicillata are able to persist in disturbed habitat especially in riparian reserves due to their opportunistic nature. Mature riparian reserves with ample recovery time are able to support otter population even in highly disturbed environment as long as there is enough shelter and prey based items.
The presence of otters was negatively associated with narrow stream, narrow stream bank and streams located far from human settlement. Otter presence was higher when the width of stream increased. Large water bodies provide wide habitat and greater availability of places for depositing spraints and have higher fish productivity (Ó Néill et al., 2009; Ruiz-Olmo et al., 2001). In addition, the increase number of density or number of otters in big rivers led to greater detection of signs (Ruiz-Olmo et al., 2001). The distance between stream edge and shoreline vegetation plays an important role to otters since they provide them with shelter, cover against predators during movement and screen disturbance along the streams (Anoop and Hussain, 2004; Khan et al., 2014; Prakash et al., 2014; Theng and Sivasothi, 2016). Riparian vegetation is known to be important for the location of holt as it provides ample security for their young from predators and not for grooming or sprainting sites. The result shows otter signs were inversely associated to stream edge distance. This is due to stream edge width of SAFE Project is generally smaller with less than 13 m. Even though vegetation cover is important to otters, they hinder grooming. Therefore, otter chooses areas with sparse to no vegetation for their fur maintenance activity (Anoop and Hussain, 2004; Shenoy et al., 2006).
The presence of otters increased when the distance of stream to human settlements decreased. This is due to otters sharing the same resources as humans, where they need both water and food resources for their livelihood. Although they tend to be shy and secretive in nature, they are usually tolerant to disturbance within their range which indicates that otters within SAFE Project are highly adaptable and can tolerate moderate to high level of human disturbances. They generally tend to avoid conflict with humans in human-dominated landscape and only come out when there is less human activity, usually at dusk or dawn. Many studies found that they are somewhat unaffected by human presence (Kanchanasaka and Duplaix, 2011). Otters mostly respond to presence of habitat characteristics used over presence of anthropogenic structures or activities in an area (Shenoy et al., 2003; Gallant et al., 2009).
CONCLUSION
In summary, this study was able to identify several important microhabitat structures correlated to the presence of otters in an oil palm dominated landscape. Due to the linear nature of their habitat, it is vital to recognize these habitat structures as a whole encompassing several habitat types. The presence of otters was higher in streams located closer to both human settlements and oil palm estates showing that the preservation of riparian reserves proved to be useful and is able to support otter population to an extent given there is ample protection, foods and habitat characteristics important for otter activity such as bank substrate and dense vegetation. Oil palm estates are able to serve as a corridor for otters to travel between reserves. Otters in the study area show a high resilience and are able to coexist with humans as long as the condition of the surrounding environment is suitable for foraging, breeding and refuge sites. When retaining riparian reserves or forested areas, it is important to make sure that all these habitat characteristics useful to otters are preserved as best as possible. Understanding the selection of habitat by otters may provide useful information towards otter conservation and management of riparian forests.
REFERENCES
Anoop, K.R., Hussain, S.A. (2004). Factors Affecting Habitat Selection by Smooth-Coated Otters (Lutra perspicillata) In Kerala, India. Journal of Zoology London. 263: 417-423.
Bedford, S.J. (2009). The effects of riparian habitat quality European Otter (Lutra lutra) in Devon. Bioscience Horizons. 2(2): 125-133.
Bhagwat, S.A., Willis, K.J., Birks, H.J., Whittaker, R.J. (2008). Agroforestry: a refuge for tropical biodiversity? Trends in Ecology and Evolution. 23: 261–267.
Cho, H. S., Choi, K. H., Lee, S. D., Park, Y. S. (2009). Characterizing Habitat Preference of Eurasian River Otter (Lutra Lutra) in streams using a Self-Organizing Map. Limnology. 10:203-213.
Danielsen, F., Beukema, H., Burgess, N.D., Parish, F., Bruhl, C.A., Donald, P.F., Murdiyarso, D., Phalan, B., Reijnders, L., Struebig, M., Fitzherbert, E.B. (2009). Biofuel Plantations on Forested Lands: Double Jeopardy for Biodiversity and Climate. Conservation Biology. 23 (2): 348-58.
Ewers, R.M., Didham, R.K., Fahrig, L., Ferraz, G., Hector, A., Holt, R.D., Kapos, V., Reynolds, G., Sinun, W., Snaddon, J.L., Turner, E.C. (2011). A large-scale forest fragmentation experiment: The Stability of Altered Forest Ecosystems Project. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 3292–3302.
Feld, C.K., Birk, S., Bradley, D.C., Hering, D., Kail, J., Marzin, A., Melcher, A., Nemitz, D., Pedersen, M.L., Pletterbauer, F., Pont, D., Verdonschot, P.F.M., Friberg, N. (2011). In: Woodward, G. (Ed.), From Natural to Degraded Rivers and Back Again: a Test of Restoration Ecology Theory and Practice, first ed. Elsevier Ltd., Amsterdam, The Netherlands.
Fisher, B., Edwards, D.P., Giam, X., Wilcove, D.S. (2011). The High Costs of Conserving Southeast Asia's Lowland Rainforests. Frontiers in Ecology and Evolution. 9 (6): 329-334.
Foster-Turley, P. (1992). Conservation Ecology of Sympatric Asian Otters Aonyx cineria and Lutra perspicillata. Ph. D. Thesis. University of Florida. pp. 172.
Foster, W., Snaddon, J., Turner, E., Fayle, T., Cockerill, T. D., Ellwood, F., Broad, G., Chung, A., Eggleton, P., Chey, V.K., Yusah, K.M. (2011). Establishing the evidence base for maintaining biodiversity and ecosystem function in the oil palm landscapes of South East Asia. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 366: 3277-3291.
Gallant, D., Vasseur, L., Dumond, M., Tremblay, E.H. Bérubé, C. (2009). Habitat Selection by River Otters (Lontra canadensis) under Contrasting Land Use Regimes. Canadian Journal of Zoology. 87: 422-432.
Gardner, T.A., Barlow, J., Chazdon, R., Ewers, R.M., Harvey, C.A., Peres, C.A., Sodhi N.S. (2009). Prospects for tropical forest biodiversity in a human-modified world. Ecology Letters. 12: 561–582.
Gray, C.L., Lewis, O.T., Chung, A.Y.C., Fayle, T.M. (2015). Riparian Reserves within Oil Palm Plantations Conserve Logged Forest Leaf Litter Ant Communities and Maintain Associated Scavenging Rates. Journal of Applied Ecology. 52 (1): 31-40.
Hon, N., Neak, P., Khov, V., Cheat, V. (2010). Food and Habitat of Asian Small-Clawed Otters in Northeastern Cambodia. IUCN Otter Specialist Group Bulletin. 27 (1):12-23.
Hussain, S.A. (1993). Aspects of the ecology of smooth-coated otters Lutra perspicillata in National Chambal Sanctuary. Ph.D Thesis. Centre for Wildlife and Ornithology. Aligarh Muslim University. Aligarh, India.
Hussain, S.A., Choudhury, B.A. (1997). Distribution and Status of the Smooth-Coated Otter Lutra Perspicillata in National Chambal Sanctuary, India. Biological Conservation. 80: 199-206.
Kanchanasaka, B., Duplaix, N. (2011). Food Habits of the Hairy-nosed otter (Lutra sumatrana) and the Small-clawed otter (Aonyx cinereus) in Pru Toa Daeng Peat Swamp Forest, Southern Thailand. IUCN Otter Specialist Group Bulletin. 28(.A): 139.- 161
Khan, W., Qasim, M., Ahmad, E., Chaudhry, A. A., Bhaagat, H. B. and Akhtar, M. (2010). Status of smooth coated otter (Lutrogale perspicillata sindica) in Pakistan. Pakistan Journal of Zoology. 42(6): 817-824.
Koh, L.P., Wilcove, D.S. (2008). Is Oil Palm Agriculture Really Destroying Tropical Biodiversity? Conservation Letter. 1: 60-64.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford: Oxford University Press. pp. 280.
Laws, A. (2016). Investigating the occupancy of otter species in oil palm and forest estates in Sabah, Malaysia. Unpublished Master’s Thesis. Durrell Institute of Conservation and Ecology (DICE), School of Anthropology and Conservation, University of Kent, United Kingdom.
Luke S.H., Barclay, H., Bidin, K., Chey, V.K., Ewers, R.M., Foster, W.A., Nainar, A., Pfeifer, M., Reynolds, G., Turner, E.C., Walsh, R.P.D., Aldridge, D.C. (2017). The effects of catchment and riparian forest quality on stream environmental conditions across a tropical rainforestand oil palm landscape in Malaysian Borneo. Ecohydrology. 10 (4): e1827. https://doi.org/10.1002/eco.1827.
MacKenzie, D.I., Nichols, J.D., Boyle, J.A., Pollock, K.H., Bailey, L.L., Hines, J.E. (2006). Occupancy Estimation and Modelling: Inferring Patterns and Dynamics of Species Occurance. Academic Press.
Malaysian Metrological Department (MMD), Ministry of Science, Innovation, Technology, Environment and Climate Change, Malaysia (2019). Eilieen Cheah to Evyen Wevan Jebrail, May 10, 2019. Letter. Series JMM/CSBH/0/600-06/5 Jld.40 (81). pp. 7.
Mason, C.F., Macdonald, S.M. (1987). The Use of Spraints for Surveying Otter (Lutra Lutra) Populations: An Evaluation. Biological Conservation. 41: 167-177.
Mitchell, S.L., Edwards, D.P., Bernard, H., Coomes, D., Jucker, T., Davies, Z.G., Struebig, M.J. (2018). Riparian Reserves Help Protect Forest Bird Communities in Oil Palm Dominated Landscapes. J. Appl. Ecol. 55: 2744–2755.
Muanis, M.C., Oliveira, L.F.B. (2011). Habitat Use and Food Niche Overlap by Neotropical Otter, Lontra longicaudis, and Giant Otter, Pteronura brasiliensis, in the Pantanal Wetland, Brazil . Proceedings of the 10th International Otter Colloquium, IUCN Otter Specialist Group Bulletin.28(.A): 76-85.
Ó Néill, L., Veldhuizen, T., Jongh, A., Rochford, J. (2009). Ranging Behaviour and Socio-Biology of Eurasian Otters (Lutra lutra) on Lowland Mesotrophic River Systems. European Journal of Wildlife Research. 55: 363-370.
Prakash, N., Mudappa, D., Shankar Raman, T.R., Kumar, A. (2012). Conservation of the Asian Small-Clawed Otter (Aonyx cinereus) in Human-Modified Landscapes, Western Ghats, India. Tropical Conservation Science. 5 (1): 67-78.
Prakash, N., Perinchery, A., Nayak, R. (2014). Monitoring otter populations and combating poaching through stakeholder participation in India (Final Report). Nature Conservation Fund. pp. 1-31.
R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 29 December 2019.
Reynolds, G., Payne, J., Sinun, W., Mosigil, G., Walsh, R.P.D. (2011). Changes in forest land use and management in Sabah, Malaysian Borneo, 1990‐2010, with a focus on the Danum Valley region. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366: 3168–3176. doi:10.1098/rstb.2011.0154.
Ruiz-Olmo, J., López-Martín, J.M., Palazón, S. (2001). The Influence of Fish Abundance on the Otter (Lutra Lutra) Populations in Iberianmediterranean Habitats. Journal of Zoology (London). 254: 325-336.
Sepúlveda, M.A., Bartheld J.L., Meynard, C., Benavides, M., Astorga, C., Parra, D., Medina-Vogel, G. (2009). Landscape Features and Crustacean Prey as Predictors of the Southern River Otter Distribution in Chile. Animal Conservation. 12: 522-530.
Shenoy, K., Varma, S., Prasad, K.V. (2003). Otters in Cauvery Wildlife Sanctuary, southern India: A study on the Habitat Selection and Diet Composition on the Smooth-coated Otter (Lutra perspicillata). Nityata Foundation, and Asian Elephant Research and Conservation Centre (A Division of Asian Nature Conservation Foundation), C/o Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
Shenoy, K., Varma, S., Devi Prasad, K.V. (2006). Factors Determining Habitat Choice of the Smooth-Coated Otter, Lutra Perspicillata in a South Indian River System. Current Science. 91(5).
Sodhi, N.S., Koh, L.P., Brook, B.W., Ng, P.K.L. (2004). Southeast Asian Biodiversity: an impending disaster. Trends Ecol Evol 19: 654-660.
Struebig, M.J., Kingston, T., Zubaid, A., Mohd-Adnan, A., Rossiter, S.J. (2008). Conservation value of forest fragments to Palaeotropical bats. Biological Conservation. 141: 2112-2126.
Struebig, M.J., Turner, A., Giles, E., Lasmana, F., Tollington, S., Bernard, H., Bell, D. (2013). Quantifying the biodiversity value of repeatedly logged rainforest: Gradient and comparative approaches from Borneo. Advances in Ecological Research. 48: 183-224.
Theng, M., Sivasothi, N., Tan, H.H. (2016). Diet of the smooth-coated otter Lutrogale perspicillata (Geoffroy, 1826) at natural and modified sites in Singapore. The Raffles Bulletin of Zoology. 64: 290-301.
Thomas, J.W., Maser, C., Rodiek, J.E. (1979). Riparian zones. In: Thomas, J. W., Maser, C. (eds.) Wildlife habitats in managed rangelands the Great Basin of southeastern Oregon. Gen. Tech. Rep. PNW-80. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station. pp. 18.
Tilman, D.J., Fargione, W.B., D'Antonio, C., Dobson, A., Howarth, R., Schindler, D., Schlesinger, W.H., Simberloff, D., Swackhamer, D. (2001). Forecasting agriculturally driven global environmental change. Science. 292: 281-284.
Relación entre la Estructura de Microhábitat y la Presencia de Nutrias en un Paisaje Dominado por Palmera Aceitera, en Sabah, Malasia
Les changements d'utilisation des terres résultant de l'expansion agricole et de l'urbanisation sont un problème de conservation mondiale en Asie du Sud-Est. L’impact de ces activités, en particulier l'intensification des plantations de palmiers à huile, ont un effet néfaste sur les habitats aquatiques, entraînant une perte de biodiversité et une dégradation de la qualité de l'eau. Les espèces liées aux cours d’eau telles que les loutres dont les habitats s'étendent linéairement au-delà des habitats non perturbés peuvent être sensibles aux paysages modifiés par l'homme en raison des changements dans les forêts et les plans d'eau environnants. La présente étude analyse la relation entre la structure des microhabitats et la présence des loutres sur base des traces de pas et des épreintes dans un paysage dominé par le palmier à huile situé dans le sud-est du Sabah, à Bornéo en Malaisie. Pour examiner la relation entre les paramètres de l'habitat et la présence des loutres, nous avons effectué une Analyse en Composante Principale (ACP) et un Modèle Linéaire Généralisé (MLG). Plusieurs structures de microhabitats se sont avérées être associées à la présence des loutres. Les résultats de l'analyse du MLG ont montré que les substrats avec un sol fortement exposé ou une combinaison de sols et de roches exposés, et un substrat à faible teneur en roches étaient corrélés à une présence plus élevée de loutres. La proximité des plantations de palmiers à huile est également un bon indicateur de la présence des loutres avec un effet positif, sur les cours d'eau situés plus près des plantations avec des indices de présence plus élevés de loutres. En revanche, la présence des loutres a été affectée négativement par l'étroitesse des cours d'eau et des rives et leur proximité de l'activité humaine. Les résultats de cette étude ont révélé la persistance des loutres dans les zones modifiées par l'homme, en particulier dans les paysages dominés par les palmiers à huile, à condition que les paramètres importants de l’habitat soient présents pour les activités des loutres (marquage, toilettage, mise bas) et pour une planification réussie de la conservation.
Revenez au dessus
Resumen: Relación entre la Estructura de Microhábitat y la Presencia de Nutrias en un Paisaje Dominado por Palmera Aceitera, en Sabah, Malasia
Los cambios en el uso de la tierra derivados de la expansión agrícola y la urbanización son una preocupación global de conservación en el Sudeste Asiático. Los impactos de estas actividades, particularmente de la intensificación de la plantación de palmera aceitera, tienen un efecto detrimental en los hábitats acuáticos, conduciendo a pérdida de bodiversidad y degradación de la calidad del agua. Las especies ribereñas, como las nutrias, cuyos hábitats se desarrollan linealmente y trascienden los hábitats no disturbados, pueden ser sensibles a los paisajes modificados por el hombre, debido a cambios en los bosques y cuerpos de agua circundantes. El presente estudio investiga la relación entre la estructura del microhábitat y la presencia de nutrias, en base a huellas y fecas/marcas olorosas, en un paisaje dominado por palmera aceitera situado en el Sudeste de Sabah, Borneo Malayo. Para examinar la relación entre los parámetros de hábitat y la ocurrencia de nutrias, condujimos un Análisis de Componentes Principales (PCA) y un Modelo Lineal Generalizado (GLM). Encontramos que varias estructuras de microhábitat están asociadas positivamente con la presencia de nutrias. Los resultados del análisis GLM mostraron que los sustratos con alta proporción de suelo expuesto o una combinación de suelo expuesto y rocas, y los sustratos con bajo contenido de rocas, soportan una más alta presencia de nutrias. La proximidad a plantaciones de palmera aceitera también es un buen predictor de presencia de nutrias, con un efecto positivo, tal que los arroyos localizados más cerca de plantaciones contenían mayor cantidad de signos de nutria. En contraste, la presencia de nurias estuvo negativamente afectada por un bajo ancho del arroyo, barrancas estrechas y arroyos localizados más ejos del asentamiento humano más cercano. Los resultados de este estudio revelaron la persistencia de nutrias en áreas modificadas por el hombre, especialmente en paisajes dominados por palmera aceitera, siempre que estén presentes parámetros de hábitat importantes para las actividades de las nutrias (marcación con fecas y marcas olorosas, acicalamiento, madrigueras) y para la planificación exitosa de conservación.
Vuelva a la tapa


