IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 37 Issue 1 (January 2020)
Citation: Wai, L., Evans, M.N., Bernard, H. and Goossens, B. (2020). Holt-Based Activity Patterns of Smooth-Coated Otter (Lutrogale perspicillata) in ihe Lower Kinabatangan Wildlife Sanctuary, Sabah, Malaysia. IUCN Otter Spec. Group Bull. 37 (1): 20 - 28
Holt-Based Activity Patterns of Smooth-Coated Otter (Lutrogale perspicillata) in the Lower Kinabatangan Wildlife Sanctuary, Sabah, Malaysia
Leona Wai1, 2, Meaghan N. Evans2, 3, Henry Bernard1 and Benoit Goossens2,3
1Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah. Malaysia
2Danau Girang Field Centre, c/o Sabah Wildlife Department, Wisma MUIS, Block B, 5th floor, 88100 Kota Kinabalu, Sabah
3Organisms and Environment Division, Cardiff School of Biosciences, Cardiff University, Sir Martin Evans Building, Museum Avenue, Cardiff CF10 3AX, UK
Corresponding Author: e-mail: leonawai22@gmail.com
Received 29th April 2019, accepted 15th June 2019
Abstract: Despite being one of the most biodiverse regions in the world, not much is known concerning the ecology of the otters on Borneo. We conducted a study to document the activity patterns of the smooth-coated otter, Lutrogale perspicillata, in increasingly disturbed and fragmented habitats in the Lower Kinabatangan Wildlife Sanctuary (LKWS), located in the Malaysian state of Sabah, northern Borneo. The aim was to gather ecological information for establishing baseline data and to understand better the otter behavior in this region of Sabah. We deployed camera traps at active otter holts, grooming and sprainting sites for 15 non-consecutive months and utilized the photographs to model the activity patterns of the otter using kernel’s density estimate modeling. Results showed that L. perspicillata in the LKWS was mainly crepuscular, with otter activity mainly occurring during early morning (0600 h) and late afternoon (1600 h - 1800 h). Grooming activity peaked at 0600 h while sprainting activity peaked at both 0800 h and 1700 h. We suggest that activity patterns of L. perspicillata may be influenced by prey availability, human disturbance and environmental temperature.
Keywords: Camera trapping, kernel density modelling, animal behaviour, Borneo
INTRODUCTION
A total of 379 species of mammals are known to be found in the island of Borneo (Phillips and Phillips, 2016), including four otter species; Lutrogale perspicillata (smooth otter), Aonyx cinereus (Asian small-clawed otter), Lutra sumatrana (hairy-nosed otter) and Lutra lutra (Eurasian otter). In Sabah, L. perspicillata and A. cinereus are commonly seen, however, no scientific studies have been conducted on otters in the Lower Kinabatangan Wildlife Sanctuary (LKWS). L. sumatrana was rediscovered in Sabah in 2010 and L. lutra was considered extinct in Borneo during the Borneo Carnivore Symposium in 2011. However, L. lutra was rediscovered and photographed in 2015.
The LKWS is a forest corridor along the Kinabatangan River in Sabah and it is an area comprised of a mixture of primary and logged lowland dipterocarp forests surrounded mainly by oil palm plantations (Abram et al., 2014; Ancrenaz et al., 2004). Despite being surrounded by human-modified landscapes, the narrow strip of forest corridor remains an important habitat for flora and fauna including otter species. To date, there are two otter species documented in the examined reaches of the LKWS; L. perspicillata and A. cinereus.
There has been no published research into the activity patterns on L. perspicillata in Borneo. In other regions within their range, the species displays diurnal behavior (Hussain, 2013), although others have reported otters will become more nocturnal following increasing levels of human disturbances (Kruuk, 2006). Camera trapping has been widely used in wildlife surveys throughout the region and has been effective in detecting elusive species such as otters (Bernard et al., 2013; Evans et al., 2016; Matsubayashi et al., 2011; Samejima and Semiadi, 2012). In this study, camera traps were used to record the daily activity patterns of L. perspicillata at active holts, grooming and sprainting sites situated within the degraded landscape of LKWS. Understanding the daily activity pattern of this species provides a valuable ecological information of L. perspicillata within degraded landscape, which will become the baseline for conserving this species in Sabah. This baseline data can be used to protect and conserve otter habitat, as well as managing human-otter conflict in Sabah.
STUDY AREA
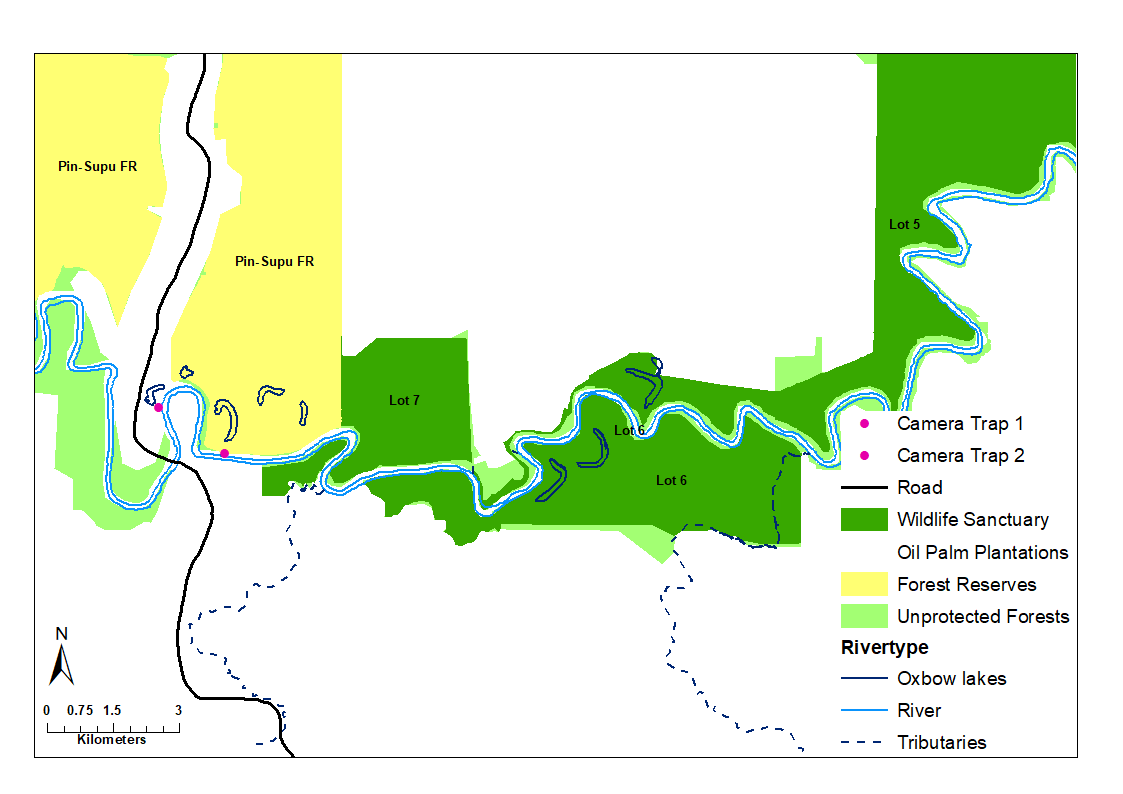
The LKWS is located on the east coast of Sabah, Malaysia Borneo (Figure 1), and comprises 27,000 ha of protected forest divided into 10 lots after being gazetted by the Sabah Wildlife Department in 2002 (Ancrenaz et al., 2004). The lowland dipterocarp forests in the Kinabatangan floodplain have undergone drastic human changes since the 1950s, particularly in the form of logging and agriculture, resulting in the extensive conversion of rainforest into oil palm (Elaeis guineensis) plantations (Abram et al., 2014; Ancrenaz et al., 2004). Despite the large amount of agriculture along the Kinabatangan River, a seemingly high diversity of Bornean species continues to persist within the floodplain (Abram et al., 2014; Evans et al., 2016). The mean annual rainfall of the region is 3,000 mm and average temperatures range from 21 - 34 °C.
METHODS
Camera trapping
A total of 40 kilometers of the Kinabatangan River (see Figure 2) were surveyed and two active otter holts, one grooming and one sprainting site were encountered. Reconyx HyperFire Professional Infrared camera traps (Models HD500 and PC800) in protective iron casings were deployed directly in front of the two active holts, an additional unit was placed at the holt grooming site, while one more was set up at the sprainting site (see Figure 2). The camera traps were set up for a period of 15 non-consecutive months (April 2016 - June 2017); monitoring was non-consecutive due to flooding events in the region, as cameras were removed to avoid damage. Camera traps took a series of three images at 1-second intervals when triggered, and during low lighting conditions, an infrared flash was used for successful and minimal stressful nocturnal imaging. Batteries and memory cards were changed and data were retrieved every 30 days
Data entry and analysis
Camera trap images were manually selected and photos not containing otters were excluded from statistical analysis. Metadata extraction was completed using ExifTool (version 9.6.8.0), which included the file name, time, date, moon phase and temperature from each selected photograph. Each burst of three images was considered a single capture, and capture events were further separated using an interval of >30 minutes between photos to avoid pseudoreplication (Vickers et al., 2017; Yasuda and Tsuyuki, 2012). Group size was disregarded for activity pattern determination; as such a photograph containing more than one otter was considered a single event. As a methodology, it is important to note that the resulting activity pattern model generated from these otter photographs represents holt-based behaviours; other activities outside the camera trap view such as hunting activity are not presented. The day-night cycle remains constant throughout the year within the study site, as sunrise occurs at 0600 h and sunset 1800 h, local time (GMT +8). Nocturnal wildlife activity in Borneo can be categorized as 1900 h - 0500 h, diurnal activity between 0700 h - 1700 h and the remaining time; 0500 h - 0700 h and 1700 h - 1900 h was categorized as crepuscular activity (Ross et al., 2013). Following the methodology of Ridout and Linkie (2009), otter activity pattern was modelled using kernel density estimates and the package ‘overlap’ (Meredith and Ridout, 2014). Statistical analyses were conducted in RStudio software (v. 3.3.3, R Core Development Team, 2018).
RESULTS
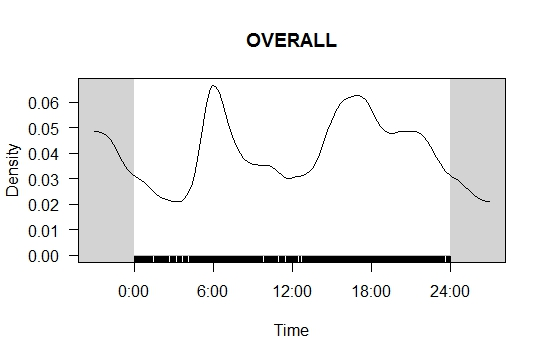
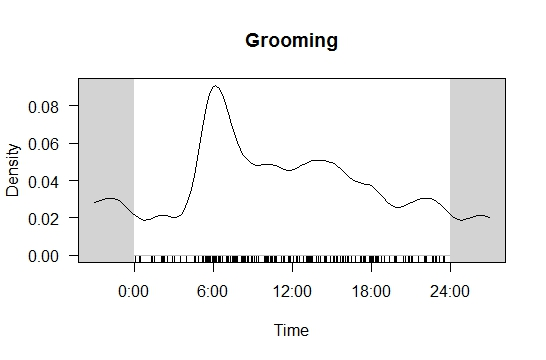
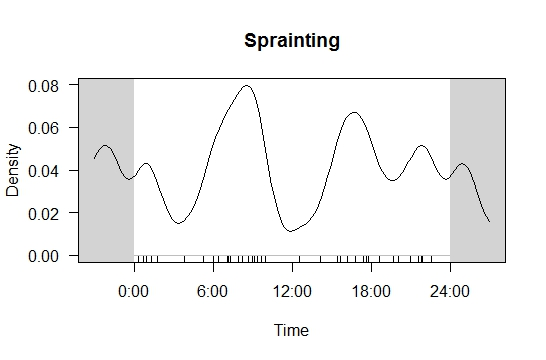
A total of 46,245 images of otters were collected from the four camera traps across 916 camera trap-nights. Based on the combined images from all four camera traps, regardless of exact position, L. perspicillata demonstrated two activity peaks; 0600 h and between 1600 h - 1800 h, which indicates that otters were most likely to be captured by the camera traps during dawn and dusk (Figure 3(a)). Moderate otter activity was recorded at 1200 h and between 1900 h - 2400 h, while the lowest activity was recorded during the night between 0000 h - 0500 h. Grooming activity pattern was generated using the same modelling parameters (see Figure 3(b)) and grooming activity peaked at 0600 h, while the lowest grooming activity recorded was between 0000 h - 0300 h. Using the same model, a sprainting site activity pattern was also generated (see Figure 3(a)). Based on the result obtained, two temporal peaks in sprainting behaviours were recorded; the first peak occurred at 0800 h and the second peaked at 1700 h. The lowest sprainting activity recorded was at 0300 h and 1200 h.
DISCUSSION
This study has produced the first documented model of the activity pattern of L. perspicillata in Sabah, Malaysian Borneo and this information will help to understand better otter behaviour in this region. The findings indicate that the holt-based activity patterns of L. perspicillata in LKWS was mainly crepuscular and this study broadly agree with Hussain (2013), who studied the same otter species in India using radio telemetry. However, these findings are in direct contrast with other L. perspicillata studies, which suggests that this species is mainly diurnal (Foster-Turley, 1992; Khan et al., 2010; Kruuk, 2006; Payne et al., 2007).
The crepuscular behavior of the Kinabatangan otters in contrast to other regions could be influenced by the differences in the availability of prey, human disturbances or ambient temperatures (Hussain, 2013). Our recorded moderate otter activity at noon might be associated with the high tropical temperatures in Sabah. High ambient temperatures will increase otter energy expenditure, directly affecting otter physiology (Anoop and Hussain, 2004; Foster-Turley, 1992; Hussain, 2013). During these temperature spikes, otters could be resting inside the holts, and therefore were not visible in front of the camera trap. Mean annual temperature in Sabah range from 25 - 30°C, and maximum temperatures are reached at midday and could exceed 32°C (Malaysian Metrological Department, 2017). However, lower temperatures (18 - 20°C) are recorded throughout the night and early morning (Malaysian Metrological Department, 2017), which could help explain our recorded otter activity peaks in the early morning. Moreover, fish, the main prey source for L. perspicillata, activity may also be affected by the high afternoon temperatures in Sabah; perhaps fish hide in cooler environments during these times and become active again when temperature is more tolerable (Hussain, 2013; Kruuk, 1995).
Contrastingly, low nocturnal temperatures may increase otter activity. However, in this study, otter activity was moderately low during the night. Perrin and Carranza (2000) reported spot-necked otter (Hydrictis maculicollis) activity was positively correlated with the detection of prey, such that low detection of fish during the night caused the otters to become less nocturnally active. The above statement suggests that L. perspicillata in the study area might be actively hunting on the river during the day when their visibility is at the best, however, hunting activities were not detected due to the location of our camera trap.
Another possible explanation that affects the otter activity pattern is the presence of saltwater crocodiles (Crocodylus porosus) in the study site. Otters may display behavioural changes to adapt with their surroundings, and this could include adapting their activity patterns to avoid predation risk from C. porosus or to minimize interspecific competition for fish. Saltwater crocodiles were documented actively hunting at night in the study area, although some satellite-tracked individuals displayed elevated activity peaks at crepuscular times (Evans, 2016). It is suggested that the increases in holt-based activity by L. perspicillata may be in response to the presence of saltwater crocodiles. Indeed, Hussain (1993) reported avoidance of mugger crocodile (Crocodylus palustris) basking sites. In other regions, otter species co-occur with a range of crocodilian species (Hussain, 2013; Kruuk, 2006; Reed-Smith et al., 2015; Ribas et al., 2012), but little is known concerning the interactions between these two river predators.
Grooming site utilisation of L. perspicillata in the LKWS was mostly recorded in the morning, and this may be associated with otter’s natural behaviour; otters groom themselves after hunting bouts. After diving under water, otters need to dry their fur to maintain its insulating ability (Hussain, 2013; Kruuk, 2006). Foraging sessions of L. perspicillata have been observed and recorded mostly in the morning and evening (Hussain, 2013; Kruuk, 1992). Therefore, our recorded increase in morning grooming activity might relate to morning hunting activity. Even though L. perspicillata have previously been reported to actively hunt during the evening, we recorded low grooming activity in the evening. The findings may suggest that L. perspicillata in the region may have multiple grooming sites and they were grooming at other sites in the evening, outside of the camera trap view.
Results from the modelled sprainting activity pattern presented sprainting peaks in the morning and evening. Similar to grooming behavior, sprainting has also been related to hunting bouts (Kruuk, 1992), which could explain the high sprainting activity in the morning and evening. Our modelled sprainting activity pattern presents as a bimodal wave across the diel except noon, which indicates that otters are constantly sprainting throughout the day. Otters are territorial mammals and often mark their territory with repetitive sprainting (Brzeziński and Romanowski, 2006; Kruuk, 1992; Shenoy et al., 2006; White et al., 2003). Our reported decrease in afternoon sprainting activity may be also due to the increase of grooming activity during that time. Other studies (Anoop and Hussain, 2004; Shenoy et al., 2006) have reported that smooth-coated otter may spraint at their grooming site. However, in this study, no spraint was detected at the grooming site, suggesting differences in behaviour of the same species in other regions.
Although camera traps have been widely used to record wildlife behaviour, there were several limitations intrinsic to the usage of this methodology in this study. Camera traps were removed several times due to flooding. It would be interesting to record otters’ behaviour and activity patterns outside their holts and associated sites during the flooding season; a waterproof camera trap could be used for such future monitoring work. In addition, activity data were limited to the behaviour that occurred within the camera trap view, and activity beyond this view was not recorded, and thus, not included in these preliminary activity models. For future work, other methods such as habituation observation studies, satellite tracking or radio telemetry, as per Hussain (2013), could be incorporated together with camera trapping to provide more detailed activity patterns for this species. Moreover, setting up the camera traps for a longer period could be useful for monitoring otter group demographics, group health and reproduction cycles over the years. Camera trapping can be easily adopted to study this species in the future, however, targeted questions and an awareness of the methodological limitations of the technology are required for effective future research. These preliminary activity patterns determined by this study serve as valuable baseline knowledge on how this species persists in Borneo, specifically in a degraded and human-modified landscape.
Acknowledgements: We are grateful to all people who have contributed directly and indirectly towards making this research a success. Sincere thanks to Sime Darby Foundation for sponsoring this project, as well as the Institute of Tropical Biology and Conservation of Universiti Malaysia Sabah and the Sabah Wildlife Department for permission to conduct this research. Deepest thanks to the Danau Girang Field Centre staff, especially Lucy, Elisa, Maz, Matt, Lee, Fab, Koko, Alut, Yusri, Daniel and Jess, as well as volunteers and students for assisting this project. We also want to express our gratitude to KOPEL Batu Puteh, Kinabatangan and the villagers of Batu Puteh for their continuous support and collaboration. Many thanks are extended to Nicole Duplaix, Jeremy Smith, and Jim Peterson for their useful ideas and guidance.
REFERENCES
Abram, N.K., Xofis, P., Tzanopoulos, J., Macmillan, D. C., Ancrenaz, M., Chung, R., Goossens, B. (2014). Synergies for improving oil palm production and forest conservation in floodplain landscapes. PLoS ONE 9: 1-12.
Ancrenaz, M., Goossens, B., Gimenez, O., Sawang, A., Lackman-Ancrenaz, I. (2004). Determination of ape distribution and population size using ground and aerial surveys: a case study with orang-utans in lower Kinabatangan, Sabah, Malaysia. Animal Conservation 7: 375-385.
Anoop, K.R., Hussain, S.A. (2004). Factors affecting habitat selection by smooth-coated otters (Lutra perspicillata) in Kerala, India. Journal of Zoology London, 263: 417-423.
Bernard, H., Baking, E.L., Matsubayashi, H., Ahmad, A.H., (2013). Records of Bornean felids in and around Tabin wildlife reserve, Sabah, Malaysia. Cat News 56: 4–7.
Brzeziński, M., Romanowski, J. (2006). Experiments on sprainting activity of otters (Lutra lutra) in the Bieszczady Mountains, southeastern Poland. Mammalia 70: 58-63.
Evans, L.J. (2015). Assessing the impacts of habitat fragmentation and subsequent anthropogenic expansion on the behavioural, nesting and population ecology of the estuarine crocodile, Crocodylus porosus. Unpublished PhD thesis, Cardiff University, 172 pages.
Evans, M.N., Vickers, S.H., Abu-Bakar, M.S., Goossens, B. (2016). Small carnivores of the Lower Kinabatangan Wildlife Sanctuary, Sabah, Borneo, including a new locality for the otter civet Cynogale bennetti. Small Carnivore Conservation 54: 26-38.
Foster-Turley, P.A. (1992). Conservation aspects of the ecology of Asian small-clawed and smooth otters on the Malay Peninsula. IUCN Otter Specialist Group Bulletin 7: 26-29.
Hussain, S.A. (1993). Aspect of the ecology of smooth-coated otter in National Chambal Sanctuary. PhD thesis.
Hussain, S.A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth-coated otter (Lutrogale perspicillata) in National Chambal Sanctuary, India. IUCN Otter Specialist Group Bulletin 30: 5-17.
Khan, W., Qasim, M., Ahmad, E., Chaudhry, A.A., Bhaagat, H.B., Akhtar, M. (2010). Status of smooth coated otter (Lutrogale perspicillata sindica) in Pakistan. Pakistan Journal of Zoology 42: 817-824.
Kruuk, H. (1992). Scent marking by otters (Lutra lutra): signalling the use of resources. Behavioural Ecology 3: 133-140.
Kruuk, H. (1995). Wild otters - Predation and populations. Oxford: Oxford University Press.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford: Oxford University Press.
Malaysian Metrological Department (MMD), Ministry of Science, Innovation and Technology, Malaysia. (2017). “Malaysia’s Climate”.
http://www.met.gov.my/web/metmalaysia/education/climate/generalclimateofmalaysia?p_p_id=56_INSTANCE_zMn7KdXJhAGe&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_pos=1&p_p_col_count=2&_56_INSTANCE_zMn7KdXJhAGe_page=7 Accessed on December 7, 2017.
Matsubayashi, H., Bernard, H., Ahmad, A.H. (2011). Small carnivores of the Imbak Canyon, Sabah, Malaysia, Borneo, including a new locality for the Hose’s civet Diplogale hosei. Small Carnivore Conservation 45: 18–22.
Meredith, M., Ridout, M. (2017). Overlap: Estimates of coefficient of overlapping for animal activity patterns. R package version 0.2.7. http://CRAN.R-project.org/package=overlap (Accessed on June 6, 2017).
Payne, J., Francis, C.M. (2007). A Field Guide to the Mammals of Borneo. Kota Kinabalu: The Sabah Society.
Perrin, M.R., Carranza, I.D. (2000). Activity patterns of spotted-necked otters in the Natal Drakensberg, South Africa. South African Journal of Wildlife Research 30: 1-7.
Phillips, Q., Phillips, K. (2016). Mammals of Borneo and Their Ecology: Sabah, Sarawak, Brunei and Kalimantan. Kota Kinabalu: Natural History Publications (Borneo).
Reed-Smith, J., Jacques, H., Somers, M.J. (2015). Hydrictis maculiocollis. The IUCN Red List of Threatened Species 2015. E.T12420A21936042.
Ribas, C., Damasceno, G., Magnusson, W., Leuchtenberger, C., Mourao, G. (2012). Giant otters feeding on caiman: evidence for an expanded trophic niche of recovering populations. Studies on Neotropical Fauna and Environment 47: 19-23.
Ridout, M.S., Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural Biological and Environmental Statistics 14: 322-337.
Ross, J., Hearn, A. J., Johnson, P. J. and Macdonald, D. W. (2013). Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. Journal of Zoology, 290: 96–106
Samejima, H., Semiadi, G. (2012). First record of Hose’s civet Diplogale hosei from Indonesia, and records of other carnivores in the Schwaner Mountains, Central Kalimantan, Indonesia. Small Carnivore Conservation 46: 1–7.
Shenoy, K., Varma, S., Prasad, K.D.V. (2006). Factors determining habitat choice of the smooth-coated otter, Lutra perspicillata in a South Indian river system. Current Science
91: 637-643.
Vickers, S.H., Evans, M.N., Abu-Bakar, S., Goossens, B. (2017). The first recorded activity pattern for the Sunda stink-badger Mydaus javanensis (Mammalia: Carnivora: Mephitidae) using camera traps. Raffles Bulletin of Zoology 65: 316-324.
Wilson, D., Reeder D. (2005). Mammal Species of the World: A Taxonomic and Geographic Reference. Baltimore: The Johns Hopkins University Press.
White, P.C.L., McClean, C.J., Woodroffe, G.L. (2003). Factors affecting the success of an otter (Lutra lutra) reinforcement programme, as identified by post-translocation monitoring. Biology Conservation 112: 363-371.
Yasuda, M., Tsuyuki, S. (2012). Comparison of mammalian communities in a human-disturbed tropical landscape in East Kalimantan, Indonesia. Mammal Study 37: 299-311.
Résumé : Modèles d'Activité de la Loutre à Pelage Lisse (Lutrogale perspicillata) Lies à la Catiche, dans le Sanctuaire de la Faune Sauvage du Kinabatangan Inférieur, Sabah, Malaisie
Bien qu’elle soit l’une des régions les plus riches en biodiversité du monde, on sait peu de chose sur l’écologie des loutres à Bornéo. Nous avons mené une étude pour documenter les schémas d'activité de la loutre à pelage lisse, Lutrogale perspicillata, dans des habitats de plus en plus perturbés et fragmentés de la réserve faunique inférieure de Kinabatangan (LKWS), située dans l'État malaisien du Sabah, au nord de Bornéo. L'objectif était de rassembler des informations écologiques pour avoir des données de base et mieux comprendre le comportement de la loutre dans cette région du Sabah. Nous avons installé des pièges photos à proximité des catiches, des sites de toilettage et d’épreintes fréquentés par la loutre pendant 15 mois non consécutifs et avons utilisé les photos pour modéliser les schémas d'activité de la loutre à l'aide d’un modèle d'estimation de la densité du noyau. Les résultats ont montré que L. perspicillata dans le LKWS était principalement crépusculaire, l'activité de la loutre se situant principalement tôt le matin (600 h) et en fin d'après-midi (1600 h à 1800 h). Les activités de toilettage ont culminé à 600 h, tandis que les activités d’épreintes ont atteint leur pic à 800 h et à 1700 h. Nous suggérons que les schémas d'activité de L. perspicillata pourraient être influencés par la disponibilité en proies, les perturbations humaines et la pollution de l'environnement.
Revenez au dessus
Resumen: Patrones de Actividad en la Madriguera, de Nutrias Lisas (Lutrogale perspicillata) en el Santuario de Vida Silvestre de Kinabatangan Inferior, Sabah, Malasia
A pesar de ser una de las regiones más biodiversas del mundo, no se sabe mucho sobrre la ecología de las nutrias en Borneo. Condujimos un estudio para documentar los patrones de actividad de la nutria lisa, Lutrogale perspicillata, en hábitats con disturbio y fragmentación creciente en el Santuario de Vida Silvestre de Kinabatangan Inferior, ubicado en el estado malayo de Sabah, Borneo del norte. El objetivo fue obtener información ecológica para establecer datos de base para entender mejor el comportamiento de las nutrias en esta región de Sabah. Desplegamos cámaras-trampa en madrigueras activas de nutria, y sitios de marcación y acicalamiento, durante 15 meses no-consecutivos, y utilizamos las fotos para modelar los patrones de actividad de las nutrias en base a modelado de densidad estimada de núcleos (kernel). Los resultados mostraron que L. perspicillata en Kinabatangan es principalmente crepuscular, concentrándose la actividad de las nutrias principalmente durante la primera mañana (0600 h) y el final de la tarde (1600 h – 1800 h). La actividad de acicalamiento tuvo un pico a las 0600 h, y la de marcación (fecas) tanto a las 0800 h como a las 1700 h. Sugerimos que los patrones de actividad de L. perspicillata pueden estar influenciados por la disponibilidad de presas, el disturbio humano y la temperatura ambiente.
Vuelva a la tapa