IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 37 Issue 1 (January 2020)
Citation: Wilson, KF and Namaskari, N (2020). Analysis of the Food-Web of a Population of Smooth-Coated Otters Lutrogale perspicillata (Mammalia: Mustelidae) in a Saline Littoral Mangrove Habitat. IUCN Otter Spec. Group Bull. 37 (1): 3 - 19
Analysis of the Food-Web of a Population of Smooth-Coated Otters Lutrogale perspicillata (Mammalia: Mustelidae) in a Saline Littoral Mangrove Habitat
Robyn, F. Wilson1* and Nityasa Namaskari2
1School of Science, Monash University Malaysia
2Bandar Sunway, Selangor, Malaysia
* Corresponding Author
Email - robynwilsonpossum@gmail.com
Received 31st March 2019, accepted 6th June 2019
Abstract: Aquaculture expansion, human-population pressure and retaliatory killing are threatening the smooth-coated otter (Lutrogale perspicillata) in mangrove habitats in Peninsular Malaysia. Our aim was to determine the diet of the smooth-coated otter (SCO) in a mangrove habitat, their feeding strategy and develop a food-web to inform the conservation of this species. We conducted spraint analysis and interviews with locals to identify the diet of SCO in the mangroves. We collected 91 spraints and identified 16 food items from six different taxa; fish, crab, shrimp, snake, barnacle and bivalve. Score bulk estimate and frequency of occurrence of prey were used to compare the importance of different taxa in the diet and this along with gut analysis of fish in the area were used to build a food-web. We found no dominate taxa but seasonal differences in their diet. SCO specialized on fish, crab and snake with fish comprising 44% and crab 43% of the diet. Fish occurred more frequently in the diet in the wet season and crab in the dry season. We conducted 25 interviews to determine tolerance of residents to SCO and to obtain feeding observations of them; no hunting was reported but SCO were disliked and harassed by fishermen and aquaculture farmers who saw them as competing for fish. The seasonal feeding strategy of SCO in mangrove habitat may have a greater effect on structuring the community than if their diet was dominated by fish. Conservation efforts need to focus on preventing future loss of mangroves; this may also reduce conflict between aquaculture farmers and otters.
Keywords: carnivore, piscivore, feeding strategy, trophic cascade, apex predator
The smooth-coated otter (SCO) Lutrogale perspicillata Gray 1865 (syn. Lutra perspicillata I. Geoffroy Saint-Hilaire, 1826) is the most common of the four species of otter found in Malaysia (Sivasothi and Nor 1994; Abdul-Patah et al., 2014; Rosli et al., 2015) but is under threat from anthropogenic activities involving land clearing and agricultural and residential development (Fig. 1). They are semi-aquatic, social-carnivores, hunting in small family groups (Helvoort et al., 1996) and are recognized as apex predators strongly influencing the structure of the food-web in habitats where they occur (Khan, 2015). Their presence in an environment can indicate its health as they are sensitive to aquatic pollution and degradation of the surrounding terrestrial habitat (Fournier-Chambrillon et al., 2004; Lemarchand et al., 2010, 2011). As a top predator, and being semi-aquatic, their disappearance from an ecosystem has a cascading effect on recruitment at different trophic levels in both the aquatic and terrestrial ecosystems leading to biodiversity loss, trophic skewing and decline in ecosystem functioning (Terborgh et al., 2001; Duffy, 2003; Sergio et al., 2008; Reynolds and Bruno, 2012). In Southeast Asia, SCO habitats are threatened predominantly by anthropogenic activities, and mangrove ecosystems in particular are under threat from shrimp farms, tourism development, residential expansion and river pollution (Hamzah et al., 2009; Fulazzaky et al., 2010). Along the west coast of Peninsular Malaysia, where the largest expanse of mangroves exists, the habitat of the SCO is also threatened by loss of habitat due to palm oil expansion, poultry farms, and municipal and industrial waste water (Fulazzaky et al., 2010).

There are two recognized groups of otters in the world based on their trophic specialisation (Timm-Davis et al. 2015). They are either mouth-oriented and primarily consume fish or hand-oriented invertebrate consumers. SCO are a mouth-oriented feeder and feed predominately on fish with minor supplements of a variety of prey including snakes, rats and birds (Khan et al., 2010; Hussain, 2013; Abdul-Patah et al., 2014; Timm-Davis et al., 2015).
Although listed as Vulnerable (IUCN 3.1) and in CITES (Appendix 2) (de Silva et al., 2015), the SCO continues to be poached for its pelt, as well as captured in the wild for sale as demand increases for young otters in the pet trade (Gomez et al., 2017). There is also increasing human-otter conflict especially with increasing fisheries and aquaculture activities throughout Southeast Asia as they compete for fish and shrimp (Naderi et al., 2017). In Malaysia, the SCO has total or complete protection under the Wildlife Conservation Act 2010. Despite this, the SCO has the status of ‘local conservation concern’ due to threats of habitat loss, water pollution and retaliatory killing (Abdul-Patah et al., 2014).
In this study we identified the diet of the SCO in mangrove habitat from spraint and responses from interviews with residents and used this information to develop a food-web. The aims of this study were to determine a) the diversity of prey in the diet of the SCO in mangroves; b) if there was a seasonal influence on the type of prey consumed, c) to construct a food-web of the SCO that live in the mangroves, and d) determine if there is conflict between members of the local community and otters where the habitat of humans and otters overlap.
MATERIALS AND METHODS
Study area
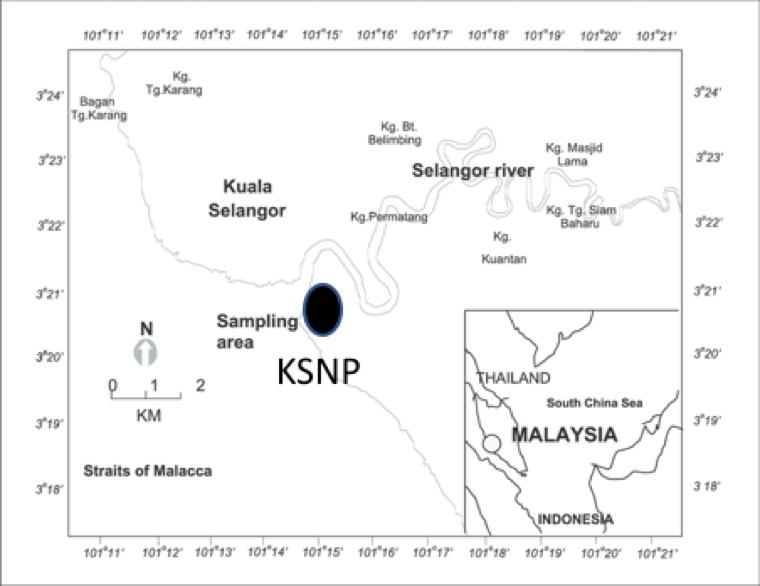
This study was conducted in the mangrove forests of Kuala Selangor (3° 20' 23.917" N, 101° 14' 14.546"E) located on the west coast of Selangor, Malaysia (Fig. 2). The coastal stretch of Selangor comprises 800,000 ha of land, of which 15,000 ha (1.6%) is covered by mangrove (Hamzah et al., 2009). The study was conducted at the Kuala Selangor Nature Park (KSNP), located in the estuary of the Selangor River; the river is highly polluted (Fulazzaky et al., 2010). The mangrove forest of KSNP is confined by a reclamation bund (dirt embankment) and channel built to drain the landward mangroves. The drained area has since modified through succession into secondary forest. A shallow man-made lake was also constructed between the secondary forest and the mangrove forest and is frequented by SCO (Davison et al., 1989). Abiotic measurements of the channels in KSNP during this study indicated they were highly polluted (mean ± SE from 30 samples taken over 8 months: turbidity 50.29 ± 6.99 cm, conductivity 8.27 ± 1.30 ms, dissolved oxygen 4.67 ± 1.01 ppm, pH 4.96 ± 0.31; water temperature 30 ± 0.6 °C. Average depth of the channels was 76.33 ± 6.99 cm).
According to the Malaysian Meteorological Department (2016), the months of August to September 2015 and February to March 2016 were particularly dry in Selangor, with average rainfall of less than 200 mm. They are referred to in this study as the Dry Season. The months of October 2015 to January 2016 had heavy rainfall (more than 400 mm), hence were considered the Wet Season.
Spraint processing
Spraint was collected opportunistically between August 2015 and the end of March 2016, along the bund wall at KSNP. Sampling was done at dawn and dusk for three days each month, except in December and January, were samples were collected over six days in both months. Only fresh spraint was collected, primarily from latrine sites where otters crossed the bund wall at dawn and/or dusk. Fresh spraint was readily identified by its wet appearance, fishy smell and for some, the presence of a green or brown mucous called anal jelly; it also consisted of predominantly fish bones and scales. Individual spraints were collected using a clean spatula, sealed in plastic bags and stored on ice for transfer to a -20 °C freezer until being processed in the laboratory. Spraint were processed by individually washing them under running water and trapping undigested components on a 1 mm sieve. Prey remnants such as bones, scales and shells were oven-dried at 60 °C for 20 - 30 minutes, weighed to obtain the total dry weight, then separated into prey classes and weighed. Remnants were examined using a Zeiss Stemi DV4 stereo microscope. Prey items in trace amounts were not included in the analysis in order to eliminate the chances of contamination, as some of the spraint were excreted on top of older spraint. The number of individuals of prey was estimated according to the observable set of otoliths, eye lens, claws, limbs, rostrum and uropods. Otoliths, backbones and scales of fish from the spraint were taken and compared with a reference sample of mature fish bought at the local fish market at Pasir Penambang, Kuala Selangor. Crabs were identified according to the rostrum and limbs and compared to a reference collection from KSNP.
For the fish reference collection, scales were taken from five different parts of the body: head, dorsum, below pectoral fin, abdomen and tail. This was to ensure variations of the scale between each body part were included in the reference sample. The fish were then dissected and gutted and the gut contents were examined under the light microscope. The remaining fish were boiled and the bones were kept in 70% ethanol. The fish were identified using either scales or otoliths. The occurrence of catfish was characterised with the presence of the spine and undigested skin. A photograph of each scale was taken to facilitate the identification of the scales from spraints.
Local interviews
Semi-structured interviews, involving the local fishermen, aquaculture farmers and residents in Kuala Selangor, were conducted to question them about the diet of the SCO and any conflict between them and otters (Appendix 1). Only locals that could correctly identify images of the SCO and had seen otters in the region were included in the survey. Qualitative data was analysed using content analysis where we grouped responses into categories and report frequency. Interviews were conducted in both English and Bahasa Melayu by Namaskari.
Analysis
Chi-square tests were used to test for differences in spraint collection between months and seasons using IBM SPSS Statistics Version 22. All testing was done with α=0.05 significance level. Score bulk estimates (SBE) and frequency of occurrence (FO) was used to determine the contribution of different food items to the diet (Fonseca et al., 2008).
Food-web metrics
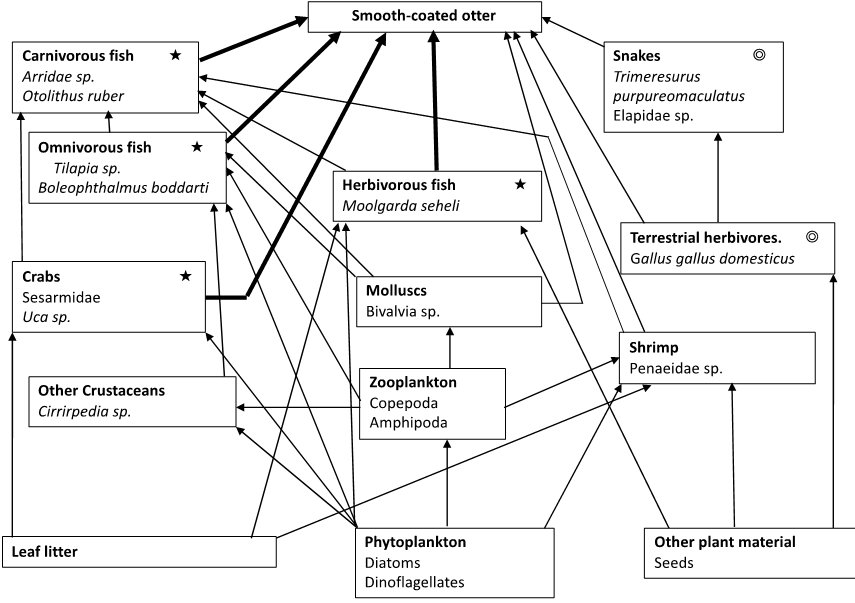
A food-web was constructed using results of the prey analyses, interviews and calculations of the
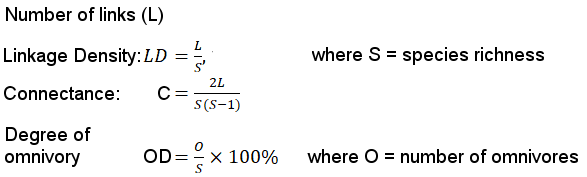
Formula for food metrics are according to Banasek-Richter et al. (2009). Higher trophic levels were built using prey items identified in the spraints, and observations of respondents we interviewed. Fish were grouped according to whether they were herbivores, omnivores, or carnivores. Lower trophic levels were constructed from gut analysis of fish from the local market in Kuala Selangor and observations of organisms in the mudflats and lake at KSNP.
Results
Number of SCO observations and spraint samples collected
SCOs were sighted 11 times at KSNP between August 2015 and March 2016. No sightings of other otter species were made or reported during the study period so it is reasonable to assume the spraint collected were from SCO. A total of 91 fresh otter spraints were collected between August 2015 and March 2016. Spraint was found during 22 of the 32 sampling days; there was a 68.8% success rate of finding at least one fresh spraint per day. The average number of spraint collected in the wet season (3.00±0.69 S.E.) and the dry (2.54±1.10 S.E.) was not significantly different (χ21=1.282, P=0.258).
Dietary composition
Six different prey taxa were found in the samples: fish, crab, shrimp, snake, bivalve and barnacle (Table 1). The most common fish was catfish Ariidae sp., followed by tilapia Oreochromis sp. The scales of five unknown species were found in the samples, one on six occasions. In three samples, neither scales nor otoliths were found. Crabs were found in 38 spraint and were mostly of sesarmid crabs; they occurred more than twice as frequently as other food items (Table 1). All the shrimp were penaeid shrimps. The snakes, identified from scales of undigested skin, were all mangrove pit viper Trimeresurus purpureomaculatus (S. Wong, personal communication February 2016).
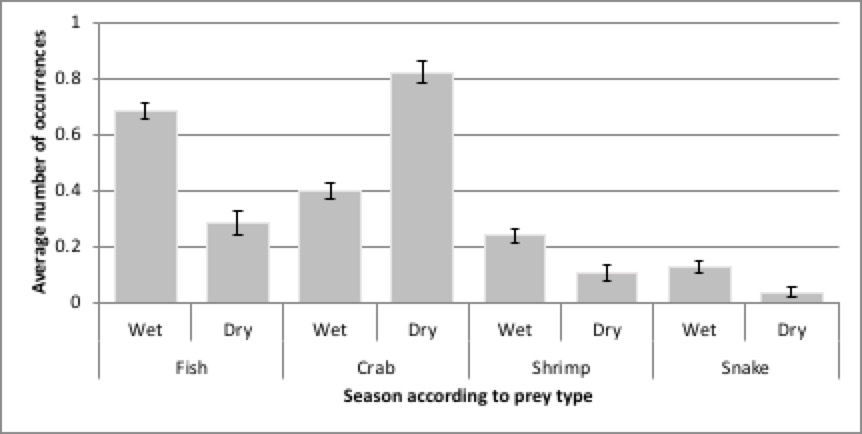
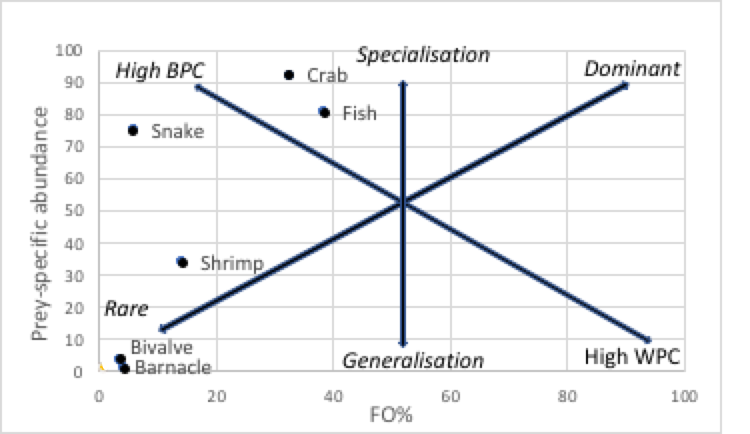
SBE revealed that the most consumed prey taxa were fish (44%) and crabs (42.68%), followed by shrimp (6.77%) and snake (5.92%), with negligible percentages of bivalve and barnacle. The feeding strategy of the SCO suggest they are specialising on crab, fish and snake whereas shrimp, bivalve and barnacle were taken opportunistically; no food taxa were dominant in the diet (Fig. 3). Niche width (high between-phenotype vs high within-phenotype contribution) indicated crab, fish and snake were high between-phenotype whereas shrimp, bivalve and barnacle were high within phenotype. Levin’s niche breadth (NB) index calculated for the SCO in this study was 3.53 and the standardised NB value was 0.51 indicating there is no dominate use of a single resource and resources are not used equally.
The average daily prey diversity was highest in November (mean=1.78, S.E.=0.11) and lowest in February (mean=1.00, S.E.=0). Five different types of prey were found in some spraint in November but only one type of prey (crab) in February. There was no significant difference in the number of prey types between the months (χ27=10.645, P=0.155). However, there was a significant difference in the average number of prey types found between the seasons (χ21=5.990, p<0.014) with a greater number of different types of prey in the spraint in the wet season (mean±S.E.=1.62±0.05), than the dry season (mean±S.E.=1.26±0.07). Fish occurrence in the spraint was much higher in the wet season than the dry whereas crabs had a higher frequency of occurrence in the dry than the wet (Fig. 4). Fish was found in high abundance throughout the sampling period except February and March; no fish were found in the diet in February, while its highest abundance was in November (mean±S.E.=0.87±0.11). Crab was found in all samples during February and March.
A total of 25 respondents were interviewed; they included 15 fishermen, six rice farmers and four aquaculture farmers, all from the Kuala Selangor region. With the exception of the aquaculture farmers, all of the respondents had seen otters in the area (i.e. channels, fishing ponds, Selangor River, paddy fields). None of the aquaculture farmers have seen otters in their ponds. Nine of the respondents, including all four aquaculture farmers, stated they didn’t like otters and they chased them when they were encountered; the rest were indifferent and ignored the otters when they sighted them. Most respondents mentioned that they have observed otters eating fish, but some also reported otters eating chicken eggs, chickens Gallus gallus domesticus, with a single sighting of an otter eating a cobra (unknown species).
Food-web metrics calculated from the food-web developed in this study (Fig. 5) included 20 species, 58 links, connectance 0.305, linkage density 2.9 and degree of omnivory 10.0%.
DISCUSSION
In this study the diversity of prey in the diet of SCO in the mangroves was relatively high, in common with other studies of SCO in more diverse habitats (Anoop and Hussain, 2005; Abdul-Patah et al., 2014; Theng et al., 2016). However, in contrast to other studies where fish comprised 69-100% of the diet of SCO (Tiler et al., 1989, Foster-Turley, 1992 (Perak, Malaysia), Kruuk et al., 1994 (Thailand); Melisch et al., 1996 (Java, Indonesia), Hussain and Choudhury, 1998 (India); Anoop and Hussain 2005 (India); Abdul-Patah et al., 2014; Theng and Sivasothi, 2016 (Singapore)) we found it did not dominate the diet of SCO in our mangrove community. Theng and Sivasothi (2016) report high levels of fish in the diet of SCO (92% SBE) when they combined their sites but when considering their mangrove site in isolation the contribution of fish in the diet was greatly reduced (65.4%). Our results similarly support the lower contribution of fish in the diet of SCO in the mangroves.
This study also found a different mix of taxa being consumed than in other studies with snake, bivalve and barnacles in the spraint but no amphibians, birds or small mammals (Table 2). This difference may reflect a difference in the availability of prey for a coastal population compared to terrestrial populations that were included in the other studies. However, barnacles and bivalves were rare in the spraint and possibly bycatch attached to other prey or scooped up along with catfish which are bottom feeders. No plant material was found in the diet in common with other studies of SCO, confirming they are obligate carnivores. The occurrence of a high percentage of crab in the diet of SCO of this study was in marked contrast to other studies that report the Frequency of occurrence (FO%) and Score Bulk Estimate (SBE%,) (Table 2). This may reflect the prey available in a mangrove habitat and the hunting conditions in the turbid water in the local system.
| Table 2: Frequency of occurrence (FO%) and Score Bulk Estimate (SBE%,) for smooth-coated otters from Anoop and Hussain (2005; India), Abdul-Patah et al., (2014; Malaysia) and Theng and Sivasothi (2016; Singapore) compared with results from this study.. | |||||||
| Prey | Frequency of occurrence (%) and Score Bulk Estimate (%) | ||||||
| This Study | Abdul-Patah et al., 2005 | Anoop and Hussain, 2005 | Theng and and Sivasothi, 2016 | ||||
| FO | SBE | FO | SBE | SBE | |||
| Fish | 38.64 | 44.00 | 72.40 | 96.02 | 92.0 | ||
| Crab | 32.58 | 42.68 | 1.00 | 1.07 | - | ||
| Shrimp | 14.39 | 6.77 | 15.00 | - | 8.0 | ||
| Snake | 6.02 | 5.92 | - | - | - | ||
| Barnacle | 3.76 | 0.33 | - | - | - | ||
| Bivalve | 4.51 | 0.31 | - | - | - | ||
| Amphibians | - | - | 3.00 | 1.08 | - | ||
| Birds | - | - | 1.00 | 1.07 | - | ||
| Insects | - | - | 1.00 | 0.76 | - | ||
| Mammals | - | - | 7.50 | - | - | ||
Levins index of the prey niche-breadth showed no dominant single source of prey being consumed, supporting the findings of Fig. 3 (FO plotted against Prey-specific abundance addressing the feeding strategy). In contrast to other studies our finding suggest the SCO were specializing on fish, crab and snake but none were dominant in the diet (Fig. 3; Table 2). Only one species of snake was identified in the spraint in this study, that was the mangrove pit viper, and may have been an opportunistic event. However, an interviewee also reported sighting a SCO consuming a snake, suggesting that snake may be important in the diet of the otter but not as readily available as fish and crabs in the mangrove habitat; this prey taxa had a lower frequency of occurrence and SBE than fish and crabs.
In this study, tilapia, an introduced species of fish to Malaysia, comprised a significant component of the diet of SCO. Consumption of this fish species by the SCO may contribute to its control but may also lead to competition with local residents who also consume and sell this fish species in the market. Anoop and Hussein (2005) also found large numbers of tilapia being consumed by SCO in Kerala, India and similarly considered SCO may control the expansion of this species.
Sesarmidae crabs were found to occur more frequently, 32 of the 91 spraints, than any other food item consumed; they were particularly important in the diet in the dry season when the SCO appeared to be specialising on them. In the dry season fish appeared less frequently in their diet and may have been less readily available in the channels and estuary due to higher temperatures affecting oxygen levels, and lower precipitation affecting salinity, water depth and nutrient concentrations, and the migration of fish out of the area (Elliott et al., 2007; Gillanders et al., 2011; Sales et al., 2018). Sesarmidae crabs were numerous on the mudflats under the mangroves at low tide throughout the study and comprise one of the highest biomasses of mangrove crabs in Malaysia (Ashton, 2002). These crabs not only make an important contribution to the diet of the SCO but also the mangrove ecosystem. In contrast, mudskippers, that were also prevalent across the mangrove flats and man-made lake at KSNP throughout the study, were not consumed as frequently as may be expected by their abundance, especially in the dry season when fish appeared less frequently in the spraint. SCO were not observed trying to excavate mudskippers from burrows in contrast to observations of this by the Asian Small-clawed otter (ASCO), Aonyx cinereus on mudflats in Bangladesh (Aziz, 2018). It may be that the ASCO that are hand-oriented invertebrate consumers are better at catching the mudskippers than the mouth-orientated SCO that are better adapted to underwater capture of prey (Timm-Davis et al., 2015).
Conflict between humans and SCOs in Malaysia was apparent from our interviews and observations of Foster-Turley (1992). However, there was no mention of the otters being hunted, killed or young taken for pets as has been reported in many localities across Asia (Hon et al., 2010; Gomez et al., 2017). There were also no reports of otters caught in nets or fish traps as has been observed in India (Kanchanasaka, 2004 in Hon et al., 2010).
It is reasonable to assume the otters are eating more items than detected in this study and thus the food-web of the SCO produced here is considered a minimalist version of their diet in a mangrove ecosystem. Further behavioural and spraint observations and the use of faecal DNA analysis stable isotopes and biologgers (Deagle et al., 2005; Rosli et al., 2014; Jeanniard-du-Dot et al., 2017) are required to improve the food-web. The latter will assist in confirming the prey items and also identify digestible items. We attempted faecal DNA analysis in this study but the results were inconclusive; we failed to get bands which could be due to several reasons including the universal primer we selected and our lack of experience in analysing faecal DNA.
There are also few studies on the SCO in Malaysia (Ratnayeke et al., 2018) and only one on the feeding strategies of SCO (Helvoort et al., 1996) who observed a group of eight SCO’s at KSNP in a coordinated feeding bout in the channel, highlighting a paucity of information on SCO.
Mangroves have been reported as an important habitat for SCO both in this study and others (Helvoort et al., 1996; Shariff, 1984 in Sivasothi and Nor, 1994; Theng and Sivasothi 2016) but are a declining resource in Malaysia where they are being destroyed in the process of land development (Hamzah et al., 2009). A recent analysis using Geographical Information Systems by Hamzah et al. (2009) of the distribution and extent of mangroves in the state of Selangor, where this study was conducted, found the mangrove habitat had decreased ‘from 28,954.6 ha in 1989 to 19,456.1ha in 2007, a reduction of about 9,498.5ha or 32.8% with the average loss of some 527.7 ha per year.’ This rate of change will affect the habitat available to and the movements of the SCO and is not sustainable for the mangrove community as a whole. As a semi-aquatic species SCO play an important role in external subsidies moving matter between habitats and their disappearance from the mangroves will also have implications for the health of neighbouring habitat (Bartels et al., 2012).
The main reason for the decline of mangroves identified by Hamzah et al. (2009) was urban, aquaculture and agriculture expansion that were also exacerbating the negative effect of tsunamis, El Niño and La Niña events. As most of these activities involve major enterprises and the government, it suggests that government agencies need to work together in managing mangroves and implementing the Permanent Forest Agreements (sustainable harvest) so no further net loss occurs.
CONCLUSION
We have shown that the diet of the SCO in a mangrove community differs from that in a terrestrial landscape; in the mangroves their diet was specialised on three taxa but no taxa dominated it. This difference in feeding strategy in the mangroves may have a greater effect on structuring the community than if the SCO focused its diet on fish as observed in other landscapes. The effect of SCO in structuring the mangrove community needs to be considered in the management of the mangrove habitat. We recommend policy development and implementation that involves the protection of the SCO, no further loss of mangroves, rehabilitation of degraded mangrove habitat and an educational program, targeting aquaculture farmers and local fishermen, that may reduce human-wildlife conflict. The latter needs to highlight the role of the SCO in the health of the mangrove community and the value of mangrove habitat in conservation.
Acknowledgements: We thank the Malaysian Nature Society and the staff at the Kuala Selangor Nature Park for allowing us to conduct this study within the park. We also thank the respondents who willingly agreed to participate in the interviews. Interviews were conducted with Monash University Human Research Ethics Committee (MUHREC) approval number CF16/907 – 2016000471. We also thank Monash University for funding to support this research. We also thank Mr. Stephen Wong for identifying the mangrove pit viper. The authors declare no conflict of interest.
REFERENCES
Abdul-Patah, P., Nur-Syuhada, N., Md-Nor, S., Sasaki, H., Md-Zain, B.M. (2014). Habitat and food resources of otters (Mustelidae) in Peninsular Malaysia. AIP Conference Proceedings 1614: 693-699.
Amundsen, P.A., Gabler, H.M., Staldvik, F. (1996). A new approach to graphical analysis of feeding strategy from stomach contents data—modification of the Costello (1990) method. Journal of Fish Biology 48(4): 607-614.
Anoop, K., Hussain, S. (2005). Food and feeding habits of smooth-coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology 266(1):15-23.
Ashton, E. (2002). Mangrove sesarmid crab feeding experiments in Peninsular Malaysia. Journal of Experimental Marine Biology and Ecology 273: 97-119.
Aziz, M. . (2018). Notes on population status and feeding behaviour of Asian Small-clawed otter (Aonyx cinereus) in the Sundarbans mangrove forest of Bangladesh. IUCN Otter Specialist Group Bulletin 35 (1): 3-10.
Banasek-Richter, C., Bersier, L.F., Cattin, M.F., Baltensperger, R., Gabriel, J.P., Merz, Y., Ulanowicz, R.E., Tavares, A.F., Williams, D.D., de Ruiter, P.C., Winemiller, K.O., Naisbit, R.E. (2009). Complexity in quantitative food webs. Ecology 90(6):1470-1477.
Bartels, P., Cucherousset, J., Steger, K., Eklöv, P., Tranvik, L.J., Hillebrand, H. (2012). Reciprocal subsidies between freshwater and terrestrial ecosystems structure consumer-resource dynamics. Ecology 93(5):1173–1182.
Costello, M.J. (1990). Predator feeding strategy and prey importance: a new graphical analysis. Journal of Fish Biology, 36(2): 261 - 263
Davis, J.C. (1975). Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. Journal of the Fisheries Board of Canada 32(12): 2295-2332.
Davison, G.W. H., Othman, M., Prentice, C., Howes, J. (1989). A coastal nature reserve in Malaysia. Oryx 23(3):138-141.
Deagle, B., Tollit, D., Jarman, S., Hindell, M., Trites, A., Gales, N. (2005). Molecular scatology as a tool to study diet: analysis of prey DNA in scats from captive Steller sea lions. Molecular Ecology 14(6):1831-1842.
de Silva, P., Khan, W.A., Kanchanasaka, B., Reza Lubis, I., Feeroz, M.M., Al-Sheikhly, O.F. (2015). Lutrogale perspicillata. The IUCN Red List of Threatened Species 2015:e.T12427A21934884.http://dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T12427A21934884.en Accessed 9 Oct 2018.
Duffy, J.E. (2003). Biodiversity loss, trophic skew and ecosystem functioning. Ecology Letters 6: 680–687.
Elliott, M., Whitfield, A.K., Potter, I.C., Blaber, S.J.M., Cyrus, D.P., Nordlie, F.G., Harrison, T.D. (2007). The guild approach to categorizing estuarine fish assemblages: a global review. Fish and Fisheries 8, 241–268.
Fonseca da Silva, V.C., Rheingantz, M.L., Fernandez, F.A. d. S. (2008). A comparison of two different methods for estimating the diet of the neotropical otter, Lontra longicaudis, with the proposal of a new index for dietary studies. IUCN Otter Specialist Group Bulletin 25 (1): 6–12.
Foster-Turley, P. (1992). Conservation aspects of the ecology of Asian small-clawed and smooth otters on the Malay Peninsula.IUCN/SSC Otter Specialist Group Bulletin 7: 26–29.
Fournier-Chambrillon, C., Berny, P., Coiffer, O., Barbedienne, P., Dasse, B., Delas, G., Galineau, H., Mazet, A., Pouzenc, P., Rosoux, R., Fournier, P. (2004). Evidence of secondary poisoning of free-ranging riparian mustelids by anticoagulant rodenticides in France: implications for conservation of European mink (Mustela lutreola). Journal of Wildlife Diseases 40: 688-695.
Fulazzaky, M.A., Seong, T.W., Masiri, M.M. (2010). Assessment of Water Quality Status for the Selangor River in Malaysia. Water Air and Pollution 205(1): 63-77.
Gillanders, B.M., Elsdon T.S., Halliday I.A., Jenkins G.P., Robins J.B., Valesini F.J. (2011). Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Marine and Freshwater Research 62: 1115-1131.
Gomez, L., Leupen, B.T.C., Theng, M., Fernandez, K., Savage, M. (2017). Illegal Otter Trade: An Analysis of Seizures in Selected Asian Countries (1980-2015) - Summary. IUCN Otter Specialist Group Bulletin 34(2): 104–114.
Hamzah, K.A., Omar, H., Ibrahim, S., Harun, I. (2009). Digital change detection of mangrove forest in Selangor using remote sensing and Geographic Information System (GIS). The Malaysian Forester 72(1): 61-69
Helvoort, B. E. van, Melisch, R., Lubis, I.R., O’Callaghan, B. (1996). Aspects of preying behaviour of smooth-coated otters Lutrogale perspicillata from Southeast Asia. IUCN Otter Specialist Group Bulletin 13(1): 3–7.
Hon, N., Neak, P., Khov, V., Cheat, V. (2010). Food and habitat of Asian Small-clawed otters In Northeastern Cambodia. IUCN Otter Specialist Group Bulletin 27(1): 12–23.
Hussain, S.A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth-coated otter Lutrogale perspicillata in National Chambal Sanctuary, India. IUCN Otter Specialist Group Bulletin 30(1): 5-16.
Hussain, S.A., Choudhury, B.C. (1998). Feeding ecology of Smooth- coated otter Lutra perspicillata in National Chambal Sanctuary. Proceedings of Symposia of the Zoological Society of London 71: 229–249. https://doi.org/10.3354/meps12381
Jeanniard-du-Dot, T., Thomas, A.C., Cherel, Y., Trites, A.W., Guinet, C. (2017). Combining hard-part and DNA analyses of scats with biologging and stable isotopes can reveal different diet compositions and feeding strategies within a fur seal population. Marine Ecology Progress Series 584: 1-16.
Khan, M. S. (2015). Occurrence of the smooth-coated otter Lutrogale perspicillata (Geoffroy, 1826) in Punjab, India. IUCN Otter Specialist Group Bulletin 32(1): 3-7.
Khan, W.A., Qasim, M., Ahmad, E., Chaudhry, A.A., Bhaagat, H.B., Akhta, M. (2010). Status of Smooth Coated Otter (Lutrogale perspicillata sindica) in Pakistan. Pakistan Journal Zoology 42(6): 817-824.
Kruuk, H., Kanchanasaka, B., O'Sullivan, S., Wanghongsa, S. (1994). Niche separation in three sympatric otters Lutra perspicillata, L. lutra and Aonyx cinerea in Huai Kha Khaeng, Thailand. Biological Conservation 69(1): 115-120.
Lemarchand, C., Rosoux, R., Berny, P. (2010). Organochlorine pesticides, PCBs, heavy metals and anticoagulant rodenticides in tissues of Eurasian otters (Lutra lutra) from upper Loire River catchment (France). Chemosphere 80: 1120-1124.
Lemarchand, C., Rosoux, R., Berny, P. (2011). Semi aquatic top-predators as sentinels of diversity and dynamics of pesticides in aquatic food webs. The case of European otter (Lutra lutra) and Osprey (Pandion haliaetus) in Loire River catchment, France. In: Pesticides in the Modern World: Risks and benefits. (Ed. Stoytcheva, M.) In TechOpen, DOI: 10.5772/17196. Available from: http://www.intechopen.com/articles/show/title/semi-aquatic-top-predators-as-sentinels-of-diversity-and-dynamics-of-pesticides-in-aquatic-food-webs
Malaysian Meteorological Department (2016). Monthly weather bulletin [online], available: http://www.met.gov.my/web/metmalaysia/publications/bulletinpreview/monthlyweather [accessed 23 April 2016].
Melisch, R., Kusumawardhami, L., Asmoro, P.B., Lubis, I.R. (1996). The otters of West Java: A survey of their distribution and habitat use and a strategy towards a species conservation programme. PHPA/Wetlands International – Indonesia Programme, Bogor, 80pp.
Naderi, S., Mirzajani, A., Hadipour, E. (2017). Distribution of and threats to the Eurasian otter (Lutra lutra) in the Anzali Wetland, Iran. IUCN Otter Specialist Group Bulletin 34(2): 84-94.
Ratnayeke, S., van Manen, F.T., Clements, G.R., Kulaimi, N.A.M., Sharp, S.P. (2018). Carnivore hotspots in Peninsular Malaysia and their landscape attributes. PLoS ONE 13(4): e0194217.
Reynolds, P.L., Bruno, J.F. (2012). Effects of trophic skewing of species richness on ecosystem functioning in a diverse marine community. PLOS ONE 7(5): e36196.
Rosli, M.K.A., Syed-Shabthar, S.M.F., Abdul-Patah, P., Abdul-Samad, Z., Abdul, S.N., Burhanuddin, M.N., Zulkifli, N.A., Shukor, M.N., Budsabong, K., Changtragoon, S., Sekiguchi, T., Sasaki, H., Md-Zain, B.M. (2014). A new subspecies identification and population study of the Asian Small-clawed otter (Aonyx cinereus) in Malay Peninsula and southern Thailand based on fecal DNA method. The Scientific World Journal, Article [457350]. DOI: 10.1155/2014/457350
Rosli, M.K., Abdul-Patah, P., Syed-Shabthar, S.M.F., Burhanuddin, M.N., Sekiguchi, T., Sasaki, H., Shukor, M.N., Yaakop, S., Md-Zain, B.M. (2015). Phylogenetic relationships of the Malay Peninsula otters (Lutra sumatrana, Lutrogale perspicillata, and Aonyx cinereus) based on DNA sequences of mitochondrial d-loop region. Journal of Animal and Plant Sciences 25(3): 836-843.
Sales, N.D.S., Baeta, A.S.B.V., de Lima, L.G., Pessanha, A.L.M. (2018). Do the shallow‐water habitats of a hypersaline tropical estuary act as nursery grounds for fishes? Marine Ecology 39(1):e12473.
Sergio, F., Caro, T., Brown, D., Clucas, B., Hunter, J., Ketchum, J., McHugh, K., Hiraldo, F. (2008). Top predators as conservation tools: ecological rationale, assumptions, and efficacy. Annual Review Ecology, Evolution, and Systematics 39: 1–19.
Sivasothi, N., Nor, B.H.M. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia 285:151-170.
Terborgh, J., Lopez, L., Nuñez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G.H., Lambert, T.D., Balbas, L. (2001). Ecological meltdown in predator-free forest fragments. Science 294: 1923–1926.
Theng, M., Sivasothi, N. (2016). The smooth-coated otter Lutrogale perspicillata (Mammalia: Mustelidae) in Singapore: Establishment and Expansion in Natural and Semi-Urban Environments. IUCN Otter Specialist Group Bulletin 33(1): 37-49.
Theng, M., Sivasothi, N., Tan, H.H. (2016). Diet of the smooth-coated otter Lutrogale perspicillata (Geoffroy, 1826) at natural and modified sites in Singapore. Raffles Bulletin of Zoology 64: 290–301.
Tiler, C., Evans, M., Heardman, C., Houghton, S. (1989). Diet of the smooth Indian otter (Lutra perspicillata) and of fish eating birds; a field survey. Journal of the Bombay Natural History Society 86: 65–70.
Timm-Davis, L.L., DeWitt, T.J., Marshall, C.D. (2015). Divergent skull morphology supports two trophic specializations in otters (Lutrinae). PLoS ONE 10(12): e0143236.
Questionnaire used in semi-structured interviews PDF (273 KB)
Résumé : Analyse du Reseau Trophique des Loutres à Pelage Lisse Lutrogale perspicillata (Mammifères: Mustélidés) dans un Habitat Littoral Salin de Mangrove
L’expansion de ’aquaculture, la pression démographique et la mise à mort par représailles menacent la loutre à pelage lisse (Lutrogale perspicillata) dans les habitats de mangroves de la péninsule malésienne. Notre objectif était de déterminer le régime alimentaire de la loutre à pelage lisse (LPL) dans un habitat de type mangrove, sa stratégie d'alimentation et de développer un réseau trophique pour éclairer la conservation de cette espèce. Nous avons effectué une analyse des épreintes et des rencontres avec les habitants pour identifier le régime alimentaire des LPL dans les mangroves. Nous avons collecté 91 épreintes et identifié 16 aliments provenant de six taxons différents : poissons, crabes, crevettes, serpents, bernacles et bivalves. L’estimation globale par score et la fréquence d'apparition des proies ont été utilisées pour comparer l’importance des différents taxons dans le régime alimentaire. Ces analyses, associées à celle du tube digestif des poissons de la région, ont été utilisées pour créer un réseau trophique. Nous n’avons trouvé aucun taxon dominant, mais des différences saisonnières dans leur régime alimentaire. LPL était spécialisée dans le poisson, le crabe et le serpent, avec 44% de poisson et 43% de crabe. Le poisson était présent plus fréquemment dans l’alimentation pendant la saison des pluies et le crabe pendant la saison sèche. Nous avons mené 25 entretiens pour déterminer la tolérance des habitants vis-à-vis de la LPL et obtenir des observations sur l’alimentation de celle-ci. Aucune chasse n’a été signalée, cependant, les pêcheurs et les aquaculteurs, qui la considèrent comme une concurrente, se plaignaient de la LPL et la harcelaient. La stratégie d’alimentation saisonnière de la LPL dans les habitats de mangroves pourrait avoir un effet plus important sur la structuration de la communauté que si leur régime alimentaire était dominé par le poisson. Les efforts de conservation doivent être axés sur la prévention de la régression future des mangroves ; Cela pourrait également réduire les conflits entre les aquaculteurs et les loutres.
Revenez au dessus
Resumen: Análisis de la Red Alimentaria de una Población de Nutria Lisa Lutrogale perspicillata (Mammalia: Mustelidae) en un Hábitat de Manglar Litoral Salino
La expansión de la acuicultura, la presión poblacional humana y la matanza retaliatoria, están amenazando a la nutria lisa (Lutrogale perspicillata) en los hábitats de manglar en Malasia Peninsular. Nuestro objetivo fue determinar la dieta de la nutria lisa (NL) en un hábitat de manglar, su estrategia alimentaria, y desarrollar una red alimentaria para ayudar con información a la conservación de esta especie. Condujimos análisis de fecas y entrevistas con gente local, para identificar la dieta de la NL en los manglares. Colectamos 91 fecas e identificamos 16 items alimentarios de seis taxones diferentes; peces, cangrejos, camarones, serpientes, percebes y bivalvos. Usamos estimaciones de rankeo visual, y la frecuencia de ocurrencia de las presas, para comparar la importancia de los distintos taxa en la dieta; y ésto junto con el análisis de contenidos estomacales de peces del área, fue usado para construir una red alimentaria. No encontramos taxa dominantes, pero sí diferencias estacionales en la dieta. La NL se especializó en peces, cangrejos y serpientes, con los peces alcanzando 44% y los cangrejos 43% de la dieta. Los peces aparecieron más frecuentemente en la dieta en la estación húmeda, y los cangrejos en la estación seca. Condujimos 25 entrevistas para determinar la tolerancia de los residentes hacia la NL, y para obtener observaciones sobre alimentación; no se informó de cacería, pero la NL no está favorecida en la visión de los residentes, y es ahuyentada por los pescadores y los acuicultores, que la ven como compitiendo por los peces. La estrategia alimentaria estacional de la NL en el hábitat de manglar puede tener un mayor efecto en la estructuración de la comunidad que si la dieta estuviera dominada por peces. Los esfuerzos de conservación deben focalizarse en prevenir la futura pérdida de manglares; ésto también puede reducir el conflicto entre los acuicultores y las nutrias.
Vuelva a la tapa