IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 1 (January 2021)
Citation: Cuculescu-Santana, M., Mason, J., Purchase, K. and Mckie, R. (2021). Outdoor Enclosure Use and Behaviour of Adult and Cub Asian Small Clawed Otters Aonyx cinereus in Summer and Winter. IUCN Otter Spec. Group Bull. 38 (1): 3 -27
Outdoor Enclosure Use and Behaviour of Adult and Cub Asian Small Clawed Otters Aonyx cinereus in Summer and Winter.
Mirela Cuculescu-Santana1*, James Mason1, Kristian Purchase2, and Rhys Mckie2
1Department of Applied Sciences, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, NE1 8ST, UK
2Wildfowl and Wetland Trust Washington, Pattinson, Washington, Tyne and Wear, NE38 8LE, UK
* Corresponding Author: Email:: mirela.cuculescu@northumbria.ac.uk
(Received 19th May 2020, accepted 15th August 2020)
Abstract: The behaviour and outdoor enclosure use of a family of Aonyx cinereus otters were investigated in summer and winter at the Wildfowl and Wetland Trust Washington center, UK. In summer, swimming and paddling (adults and cubs) and diving (adults) were recorded significantly more frequently than in winter, correlated with significantly higher frequencies of use of the water features. For the cubs, the relative frequency of diving was significantly lower compared to winter, as the cubs were still learning to swim and forage underwater. The levels of activity and the diversity of behaviours were higher around feeding times in both seasons. The cubs were already swimming in shallow water at 3.5 months-old and in deeper water at 4.5 months-old, mostly as a family group. At 3.5-6 months-old they were out of sight in the den significantly more frequently than the adults and displayed more play-fighting. By 8-9.5 months-old they moved around independently, foraging or playing and their behavioural budget was similar to that of the adults. Object juggling and vigilance standing were displayed from around 4 months-old, when weaning also occurred. The introduction of additional structural enrichment (logs, holt, nest-box) in early autumn increased the frequency of use of ground areas in winter, when the water temperatures were below 10 °C. The feeding and structural enrichment strategies used were effective for keeping the otters active outdoors and maintaining their high display value in the cold season (day time summer air temperatures 15-27 °C > winter 3.5-10 °C), emphasizing the importance of enrichment for good welfare.
Keywords: activity patterns, cub development, mating behaviour, structural enrichment, thermoregulation
INTRODUCTION
Asian small clawed otters (ASCO) have been listed as Vulnerable on the IUCN Red List since 2008 and are at risk of becoming extinct in some regions in central Asia due to extensive habitat loss, water pollution and exploitation by humans (Wright et al., 2015). Field surveys suggest that the global population of ASCO has declined by more than 30% over the last 30 years (Wright et al., 2015) and their geographical range is predicted to shrink by 17-41% by 2050 due to loss of suitable climatic areas (Cianfrani et al., 2018). Their current range covers a large part of southern and south eastern Asia and includes diverse habitats, ranging from coastal and freshwater wetlands to paddy fields, rivers and lakes in forested areas at higher altitudes (Rosli et al., 2014). Their increased popularity on social media led to an increase in illegal pet trade (Kitade and Naruse, 2018; Harrington et al., 2019) and the species has been recently moved from CITES Appendix II to Appendix I (CITES, 2019).
The ASCO Survival Plan (Foster-Turley, 1986) encouraged more research into reproduction in captivity, to support the increased conservation efforts in situ and ensure long-term survival of the species both in captivity and in the wild (Bateman et al., 2009). ASCO adapt well to living in zoos and aquaria and are the most common captive otter species in the United Kingdom (Wright, 2003) and in many other countries outside their natural range (Kruuk, 2006). They are reproductively active all year round, with mating lasting for up to 30-45 minutes and occuring mostly in shallow water (Reed-Smith and Polechla, 2002; Heap et al., 2008). Reproductive success varies, with no offspring produced in some zoos (Foster-Turley, 1990). Various zookeeper accounts (AZA, 2009) and a recent quantitative study showed that ASCO spend more time swimming in warmer water (Cuculescu-Santana et al., 2017). As tropical warm-adapted mammals, they have a significantly higher metabolic rate in cold water compared to other otter species (Borgwardt and Culik, 1999), possibly due to poorer insulative capacity of the fur and skin and greater heat loss over the whole body surface compared to the cold-adapted Eurasian otter Lutra lutra and similar in pattern to that of another tropical species, the giant otter Pteronura brasiliensis (Kuhn, 2009; Kuhn and Meyer, 2009).
Many North American zoos keep ASCO in climate-controlled environments with ambient temperatures of 21-23 °C, while some institutions heat only the pool water, or keep the otters mostly inside a heated indoor shelter during the cold season (Reed-Smith and Polechla, 2002). The majority of zoos in Europe reported housing ASCO in outdoor enclosures with water features or in indoor-only enclosures, without giving specific details of climate-control (Dornbusch and Greven, 2009). The recommended temperatures for ASCO are 22.2-24.4 °C for air and 18.3-29.4 °C for water and the otters usually cope well with lower temperatures, even below 10 °C, provided they have dry shelter and a source of radiant heat in the cold season (Heap et al., 2008). However, activity levels were lower and the frequency of abnormal repetitive behaviours (ARBs) was higher in the cold season, in correlation with the increased energetic demands of thermoregulation (Cuculescu-Santana et al., 2017).
Although no single factor was identified to have a strong correlation with successful reproduction of ASCO in captivity (Reed-Smith, 1998), maintaining well-balanced energy budgets by manipulating the enclosure temperatures and feeding strategies represents an important aspect of otter husbandry (Heap et al, 2008). The introduction of other types of environmental enrichment to stimulate naturalistic active behaviours such as swimming, foraging and social play can also have an important role in fostering high welfare and increasing the likelihood of successful cub birth and development to adulthood (Hussain et al, 2011). In captivity, both parents look after the cubs, often with help from older siblings (Nair and Agoramoorthy, 2002). Cub development from birth to weaning is well documented for captive otters and seems to reach a critical stage around 14 weeks old, when weaning usually begins (Timmis, 1971; Prima, 1992; AZA, 2009). Several cub deaths around this time were reported in the earlier scientific literature, due to inability to progress acquiring solid food (Leslie, 1971; Lancaster, 1975; Sivasothi, 1998) and in some cases labour-intensive hand-rearing interventions were required (Webb, 2011). There are very few descriptions of ASCO cub behaviour beyond this stage (Owen, 2004; Wright 2005; Lemasson et al., 2014), as they become gradually more independent of their parents, and not much is known about what happens in the wild (Kruuk, 2006).
The initial aim of this study was to investigate the influence of seasonal variations in temperature on the behaviour of a pair of ASCO in an outdoor enclosure at the Wildfowl and Wetland Trust (WWT) Washington (England) and the effectiveness of adding new structural enrichment at keeping the otters active during the cold season. The range of temperatures experienced in the outdoor enclosure was expected to be broader than that experienced by the ASCO studied in an indoor enclosure at a different establishment in 2013 (Cuculescu-Santana et al., 2017) and lead to greater differences in levels of activity between summer and winter and in the use of water features. However, the existing feeding strategies and the new structural enrichment were expected to sustain the otters’ interest in exploring the outdoor areas during the colder season, keep them active and maintain their display value (AZA, 2009). The pair had already reproduced successfully in spring 2015 (one female cub), and the arrival of a new litter of four cubs in March 2016 offered the additional opportunity to study cub behavioural development from 3.5 to 9.5 months old.
METHODS
Otters, Enclosure and Enrichment
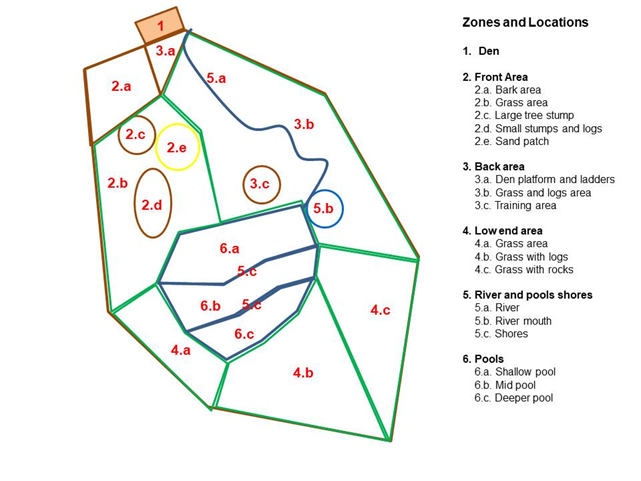
The family of ASCO studied consisted of the adult pair (captive born 6-years old male and 5 years-old female, around 3 Kg weight), a 1-year old female cub (fully grown), and four new cubs (3,1) born in March 2016 (Figure 1). The otter enclosure (approximately 400 m2 surface area, 5:1 land:water) had a variety of natural substrates outdoors (soil, grass, vegetation, bark chippings and sand) (Figure 2) and structural enrichments (several logs and tree stumps, tunnels, river and three pools with filtered recirculating water, ranging from 0.2-0.5 m depth) as well as a split-level den with a heat lamp (Figure 3).
Figure 1. The family of otters at the WWT Washington Centre, UK: (a) Top left, adult otters, Musa (M) and Mimi (F) (Photo Copyright: Mirela Cuculescu-Santana); (b) Top right, 1-year old cub, Ruby (F) (Photo Copyright: WWT); (c) Bottom, otter cubs, born 3/03/2016: Ash (F), Tod, Pip and Sam (M) (Photo Copyright: Ian Greneholt)
Figure 2. The otter enclosure at the WWT Washington Centre, UK: a) Left, den and outdoor area with structural enrichments and natural vegetation (Photo Copyright: Mirela Cuculescu-Santana); b) Right, aerial view (Google Maps, 2016).
Figure 3. Closer view of some of the structural enrichments in the otter enclosure at WWT Washington: (a) Tall tree stump with climbing ‘steps’ and tunnels in the base; ladders and platform around the entrances to the den; (b) Training area with seven logs; (c) Vegetation and stone structures around river mouth and shallow pool (Photos Copyright a-c: Mirela Cuculescu-Santana).
The otters were fed four times a day (9:00 am, 11:30 am, 3:00 pm and 5:30 pm), on a varied diet of fish, shellfish, day-old chicks and red meat, boiled eggs once a week and chopped vegetables (approximately 600g/ adult otter/ day in total, all year round). Feeding enrichment strategies included varying the form of delivery of the main feeds (scattered or hidden in different areas of the enclosure, to encourage foraging, or keeper-fed during training; food hidden inside larger vegetables, etc.) and the type of food. Public talks took place twice daily, combined with the 11:30 am and 3:00 pm feeds (Washington Wetland Centre, 2016). New structural enrichments were added to the back area and the low end area in early autumn 2016 (Figure 4 a, b) and more space was created around an existing logs structure in the back area (Figure 4 c).
Figure 4. New structural enrichments added to the back area and low end area of the otter enclosure before winter 2016: (a) nest box; (b) small holt and (c) logs structure at the back of the enclosure and nest box; trimmed vegetation gave better access to the logs structure (Photos Copyright a-c: Mirela Cuculescu-Santana).
Data Collection
Data were collected in summer 2016 (June-August; temperatures: air 15-27 °C, water 15-25 °C) and winter 2016 (November-December; temperatures: air 3.5-10 °C, water 3.5-8 °C) on mostly dry days (occasional light rain; no persistent snow cover in winter 2016).
The behaviour and the enclosure use were recorded on five different days in each season, using continuous observation and scan sampling, with one-zero recording rule for every 2-minutes interval (Rees, 2015), for 5.5 hours each day (10:30-16:00), split into 30 minutes observation periods (5 days x 11 observation periods per day x 2 seasons = 110 data sets in total, for each age group) by the same observer, separately for the adults and for the 2016-born cubs, using the ethogram shown in Table 1 and the coding system and grouping for the enclosure zones shown in Figure 5. At the same time, a second observer used instantaneous time sampling every 30 seconds to record the time spent in the water by the adult male and the female 2016-born cub.
| Table 1: Asian small-clawed otter ethogram (Adapted from Cuculescu-Santana et al., 2017). Asterisks represent categories and behaviours that may include abnormal repetitive behaviours. | |||||
| Category | Behaviour | Description of Behaviour | |||
| Land Locomotion | Walking, Running & Climbing | Faster or slower locomotion on the ground, on other flat structures or climbing on higher structures, with head up. | |||
| Swimming and Paddling | Swimming, Paddling | Locomotion in deep or shallow water, with head out of water. | |||
| Diving | Diving | Locomotion in deep or shallow water, with head under water; Foraging in the water. | |||
| Foraging and Digging | Foraging, Digging | Moving on land with the head down and the nose close to the ground; Using paws to move vegetation, dig into substrate or claw at a wooden structure. | |||
| Scent Marking | Scent rubbing, Sprainting | Rubbing a body part against a substrate or structure; Urination or defecation; Spreading faeces with the tail | |||
| Play Fighting | Social play | Chasing and tumbling together on land or in water. | |||
| Social Affiliative | Body rubbing, Social grooming, Food sharing, Parental care, Sexual behaviour, Keeper interaction | Close body contact; Using paws or mouth to clean, dry or smooth the fur of another otter; Giving food to another otter; Suckling, nudging, carrying cub; Adults holding on to each other and rolling around in or out of water, in close body contact; male chasing and mounting the female; Watching, following, nudging, biting keeper. | |||
| Aggression* | Fighting, Biting*, Snorting, Snarling | Rough fighting, with biting, shoving, hair plucking and/or scratching; Aggressive displays towards other otters. | |||
| Eating and Drinking | Eating, Drinking | Biting, chewing, handling food; Drinking water; | |||
| Solitary Activity* | Self-grooming, Playing with object, Pacing*, Abnormal behaviours*, Yawning | Using paws or mouth to clean, dry or smooth own fur, or rolling on grass or sand; Handling an object other than food (pebble, shell, straw, twig, enrichment item); Moving repetitively along the same route; Head flips, tail sucking or biting, etc.; Opening the mouth wide to take in air (presumed involuntary action). | |||
| Vigilance* | Looking around, Standing on hind paws, Begging* | Being alert and looking around from four-paws position, with head up; or Turning in a circle to scan surroundings*; Standing on hind paws to look around; Performing repetitive up-down ‘begging’ displays*. | |||
| Vocalising | Short calls*, Long squeals, Soft calls | Short, sharp and loud squeals; Longer and higher pitched loud sounds; Low intensity squeaks, chirps and chuckles. | |||
| Resting | Resting | Lying down with head down outside | |||
| Out of Sight | Out of sight | Being inside the den or holt or hidden in the vegetation. | |||
The approximate visitor numbers were also recorded for each 2-minute interval using a ranked score from 1 to 5 (1 = only 1-2 people present; 2 = 3-4 people; 3 = 5-10 people; 4 = 11-20 people; 5 = more than 20 people). Air and water surface temperatures were recorded daily using a hand-held INFRARED DT8550 thermometer.
Data Processing and Data Analysis
The behaviour and enclosure use data collected using scan one-zero sampling were processed as frequency of recording of each behaviour and each enclosure zone per observation period, grouped into behavioural categories (Table 1) and enclosure zones (Figure 5) and converted into percentages of all behaviours/locations recorded per observation period. The duration and the number of bouts of sexual behaviour and of swimming were calculated for each season. Visitor data were processed as average scores per observation period.
Average relative frequencies (%) were calculated for each age group, season and time of day. The data for cubs were also averaged by cub age in months, from 3.5 to 9.5 months-old. Non-parametric tests of difference were carried out in SPSS V26, at level of significance P<0.05 (Mann-Whitney U-test for differences between summer and winter and between adults and cubs; Kruskal-Wallis Test for the influence of time of day and cub age). The seasonal influence on the frequency of swimming bouts of each duration was tested using Pearson’s two-way Chi-square test in Microsoft Excel.
Ethical Considerations
The project received ethical approval from the Ethics Commission of Northumbria University and was carried out with consent from and in collaboration with the Collections Manager and the Keepers at the WWT Washington Wetland Centre. The data were collected from outside the enclosure, without any direct interaction between observer and otters.
RESULTS
Seasonal and Enrichment Influence
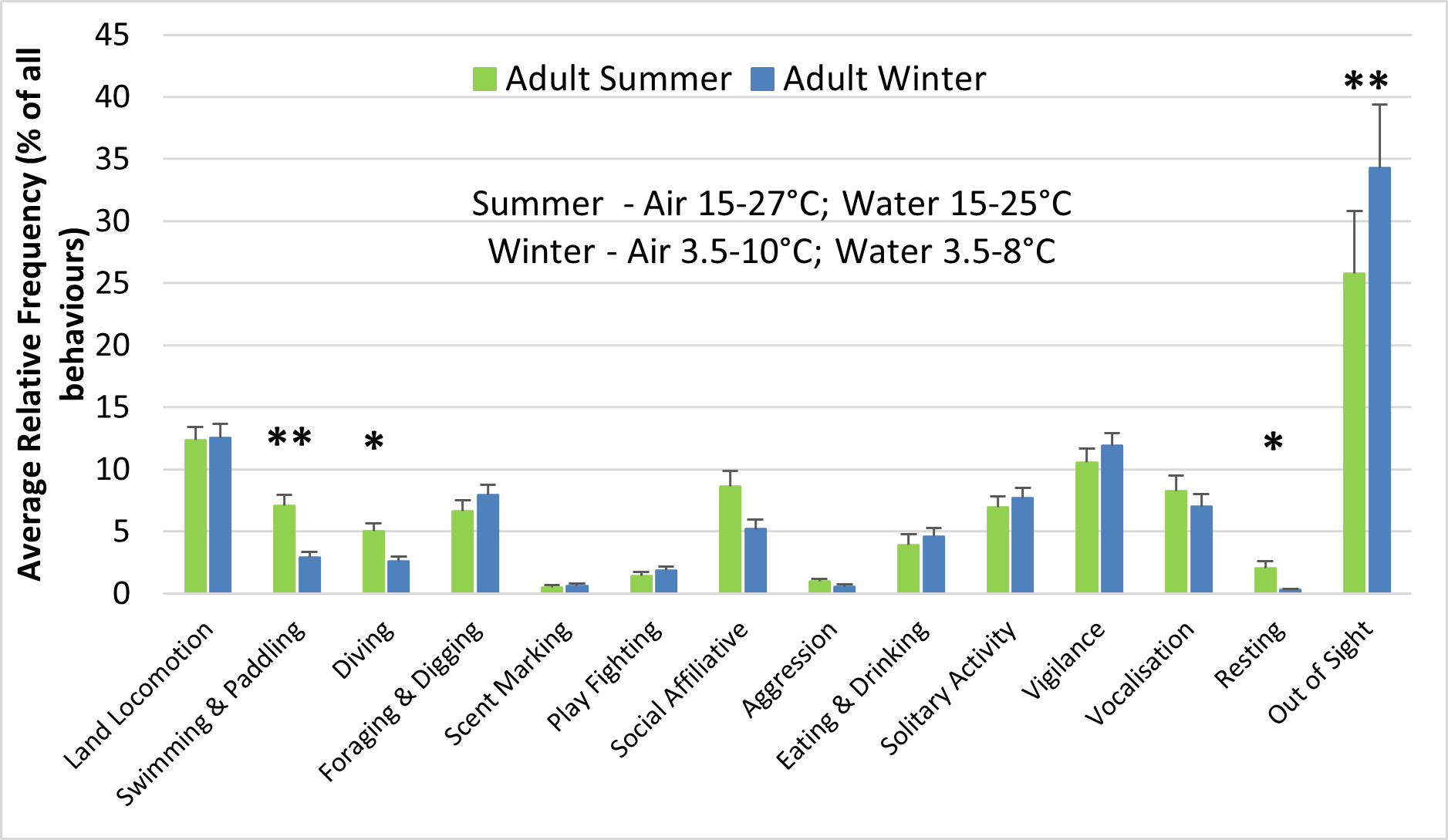
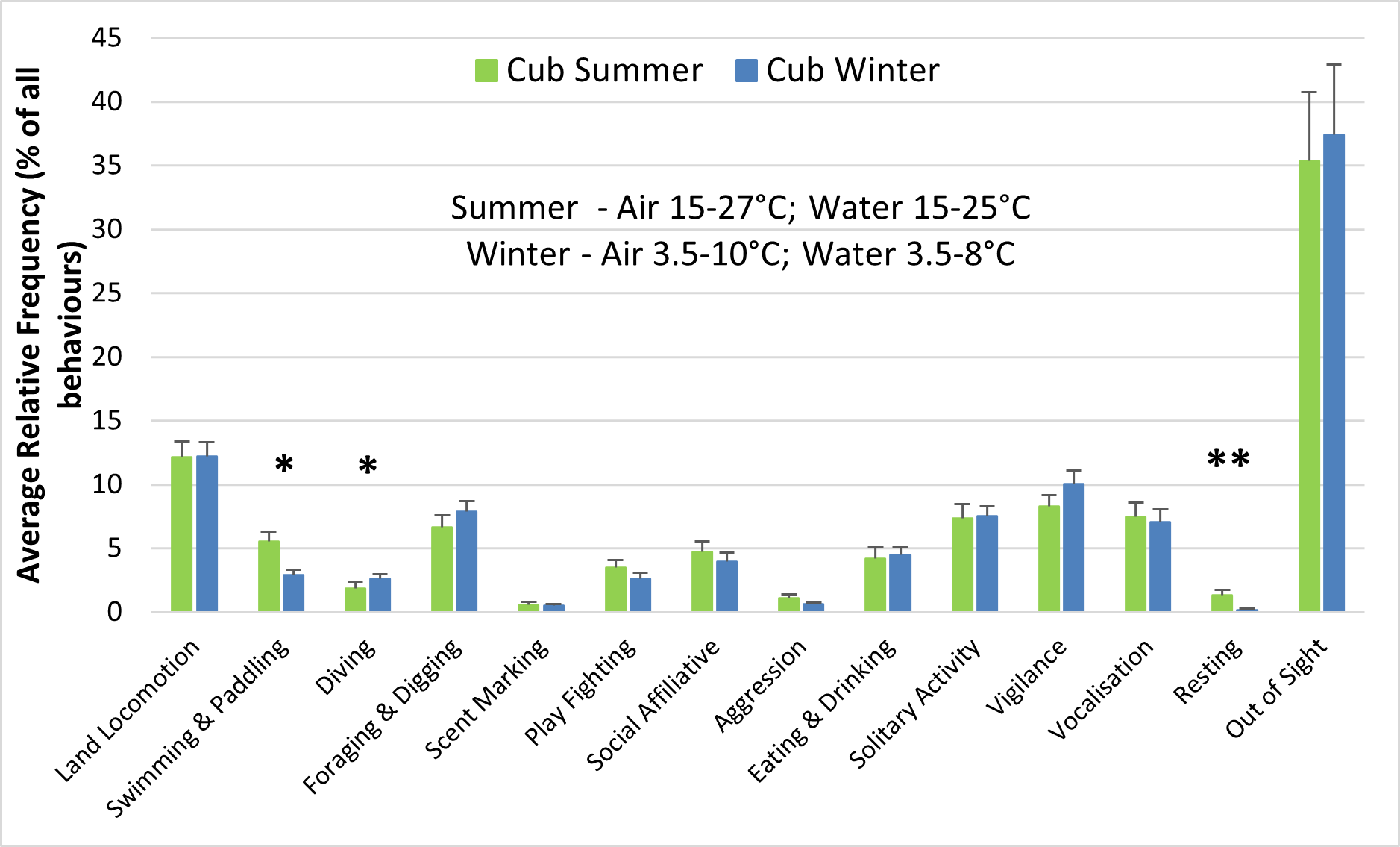
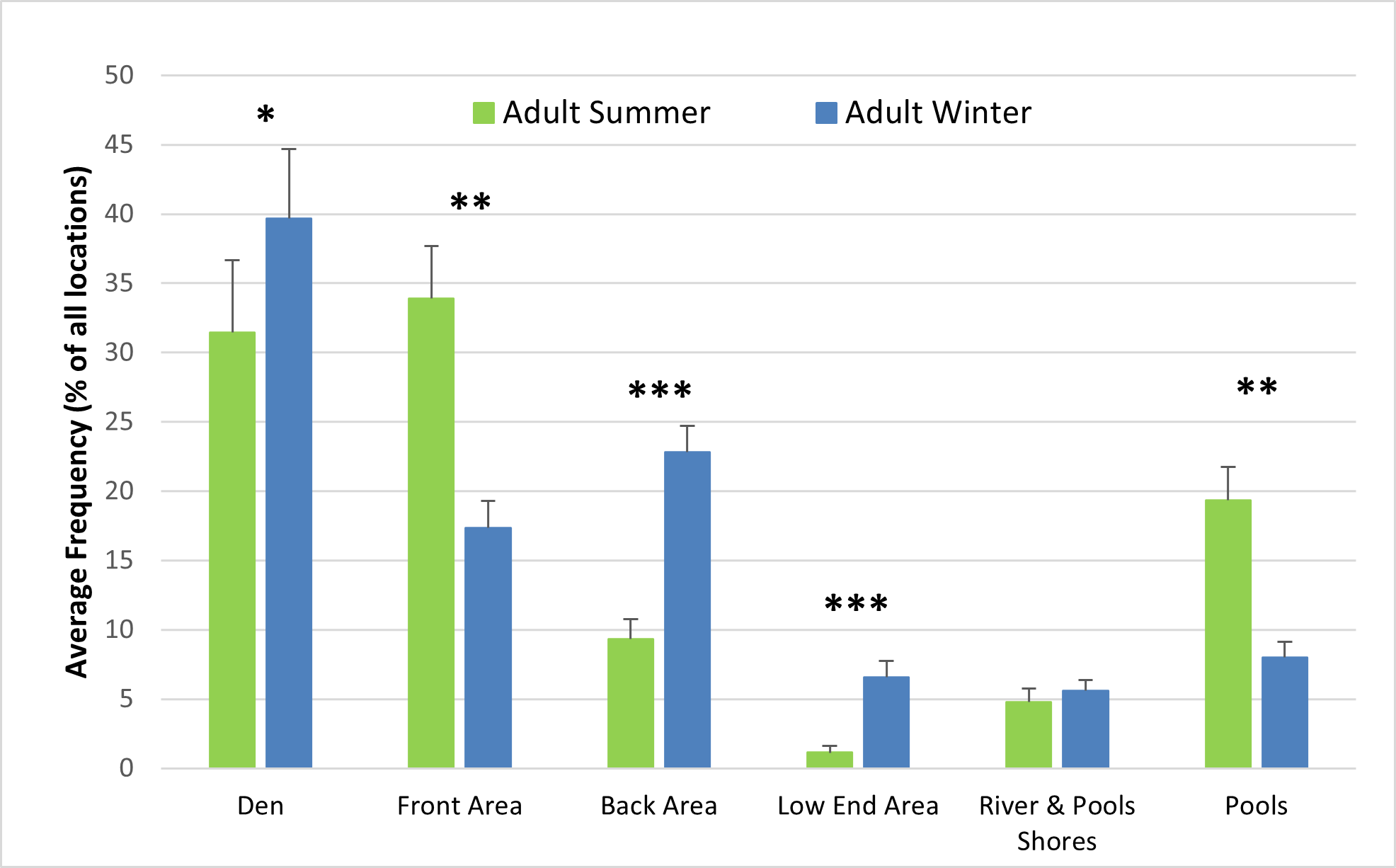
The behaviour and enclosure use of both adult otters and otter cubs were significantly influenced by seasonality (Figures 6-8). The air and water surface temperatures recorded in winter were lower than those recorded in summer (Figure 6). For the adults, swimming and paddling (7.1% > 2.9%; Z = -3.352; P<0.01), diving (5% > 2.6%; Z = -2.439; P<0.05) and resting outside (2% > 0.3%; Z = -2.321; P<0.05) represented a significantly higher percentage of all behaviours recorded in summer, compared to winter (Figure 6a). Similar differences between summer and winter were observed for the cubs for swimming and paddling (5.5% > 2.9%; Z = -1.990; P<0.05) and for resting outside (1.3% > 0.2%; Z = -2.656; P<0.01), while diving was significantly more frequent in winter (2.6% > 1.9%; Z = -2.361; P<0.05) (Figure 6b). Swimming bouts of 4 minutes or more occurred significantly more frequently than expected in summer than in winter (P<0.001; χ2=37.216), when the majority of swimming bouts lasted 1 minute or less for both the male adult and the female cub (Table 2). Social affiliative behaviours were also more frequent in summer for the adults (8.6% > 5.2%).
 b) Cubs
b) Cubs
For adults, the largest increase in relative frequency from summer to winter was seen for being out of sight (25.8% < 34.3%; Z = -2.803; P<0.01) (Figure 6a) A small increase was seen for out of sight for cubs (35.4% < 37.4%) (Figure 6b). In summer, eating frequently took place in shallow water, immediately after finding a piece of food, while in winter the otters foraged in the water but usually ate out of water (Figure 7).
Figure 7. Seasonal changes at WWT Washington: (a) summer: swimming, diving, foraging, eating and playing in the water; (b) winter: eating out of water, on the mid pool shore; (c) winter: eating out of water, on the pool shore at the low end of the enclosure (Photos Copyright a-c: Mirela Cuculescu-Santana)
In summer, the adult otters were seen diving (5% > 1.9%; Z = -3.860; P<0.001) and displaying social affiliative behaviours (8.6% > 4.7%; Z = -2.468; P<0.05) significantly more frequently than the cubs. The cubs were out of sight significantly more frequently than the adults (35.4% > 25.8%; Z = -2.198; P<0.05) and engaged in play fighting more frequently than the adults (3.5% > 1.4%). Similar differences between adults and cubs were seen in winter, but of much smaller magnitude (Figure 6).
The differences in behaviour between summer and winter and between adults and cubs were also reflected in the differences in enclosure use. Both adults and cubs used the pools (adults Z = -3.309; p<0.01; cubs n.s.) and the front area (adults Z = -2.970; P<0.01; cubs Z = -1.980; P<0.05) more frequently in summer than in winter. In winter, both adults and cubs used the den (adults Z = -2.158; P<0.05; cubs n.s.), the back area (adults Z = -4.736; P<0.001; cubs Z = -3.976; P<0.001) and the low end area (adults Z = -3.957; P<0.001; cubs Z = -4.601; P<0.001) more frequently than in summer (Figure 8), (n.s. = not significant at P<0.05 level).
 b) Cubs
b) Cubs 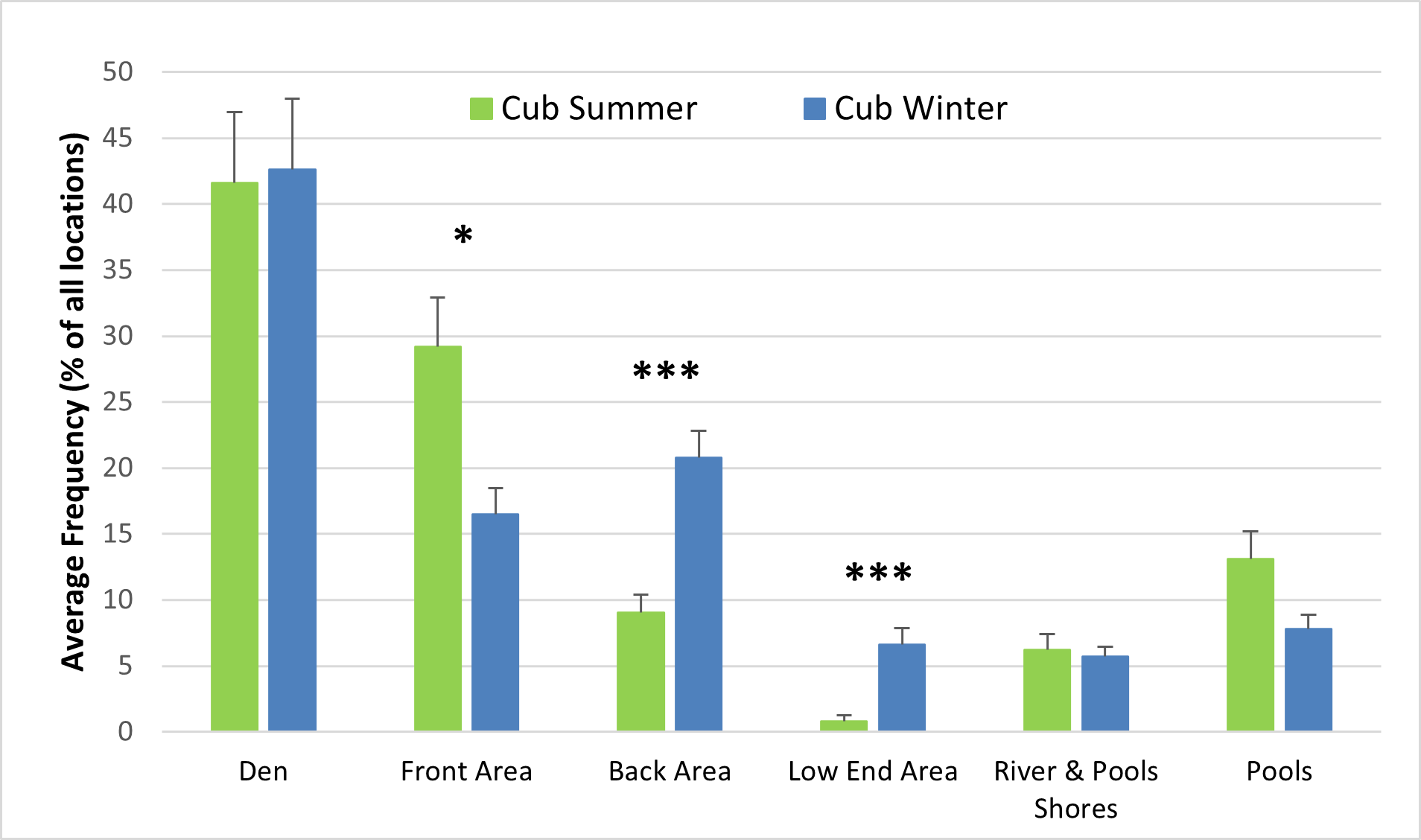
Activity Patterns
In both seasons, the otters were more active and displayed a greater variety of behaviours just before, during and just after feeding times (Figures 9,10 for adults, very similar patterns for cubs, not shown). Land locomotion, swimming, vigilance and vocalisations increased just before feeding times, while behaviours such as eating and drinking, foraging and digging and diving increased during feeding, as expected. Aggression was also recorded more frequently during feeding. Play fighting and other social affiliative behaviours (eg grooming, body rubbing) and solitary behaviours (self-grooming, playing with objects) increased in frequency during the 30-min intervals just after those during which feeding occurred. The frequency of recording of many behaviours was significantly influenced by time of day, especially in summer (Kruskal-Wallis test, P<0.05 level of significance; see captions of Figures 9-10).
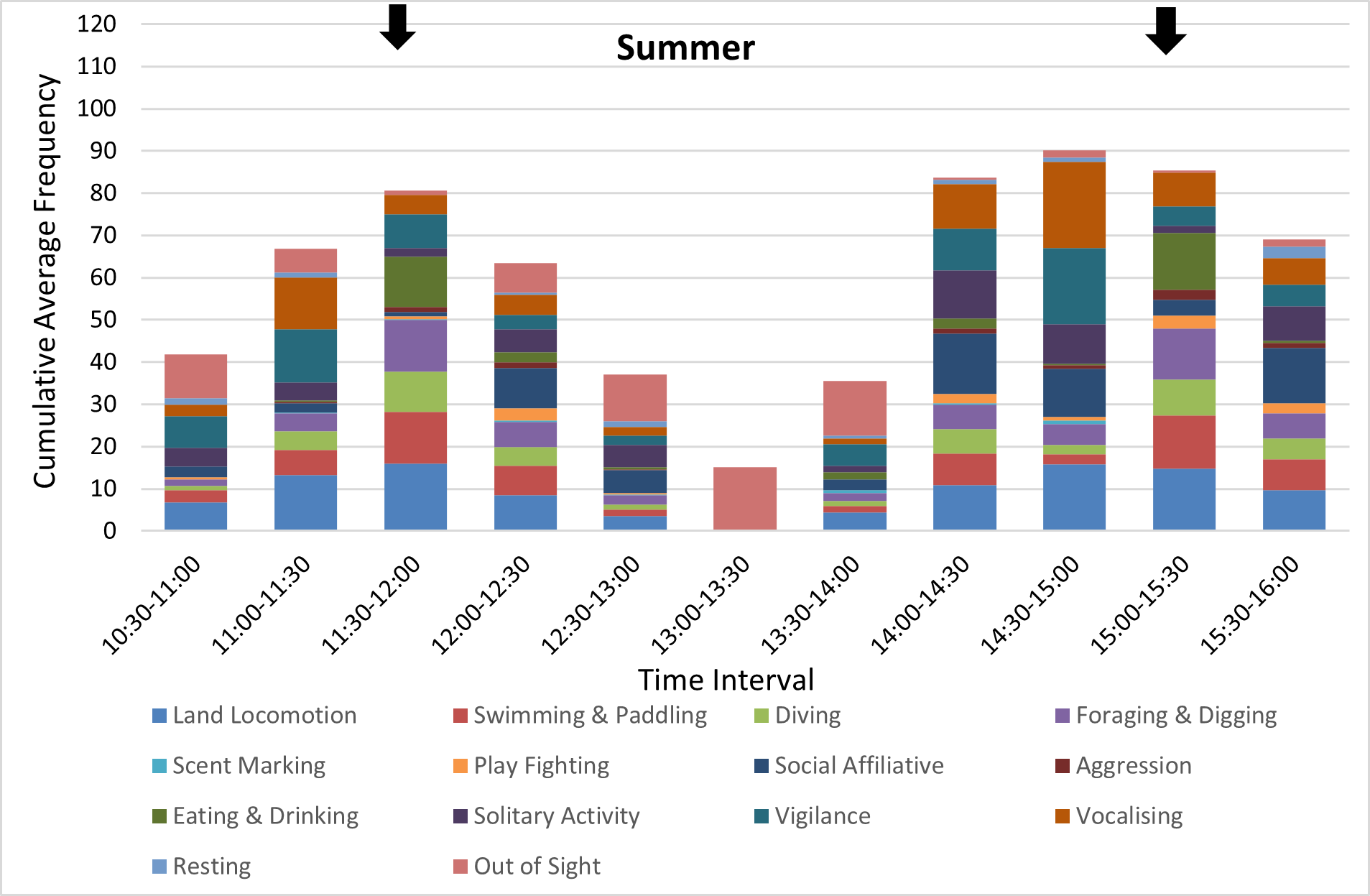
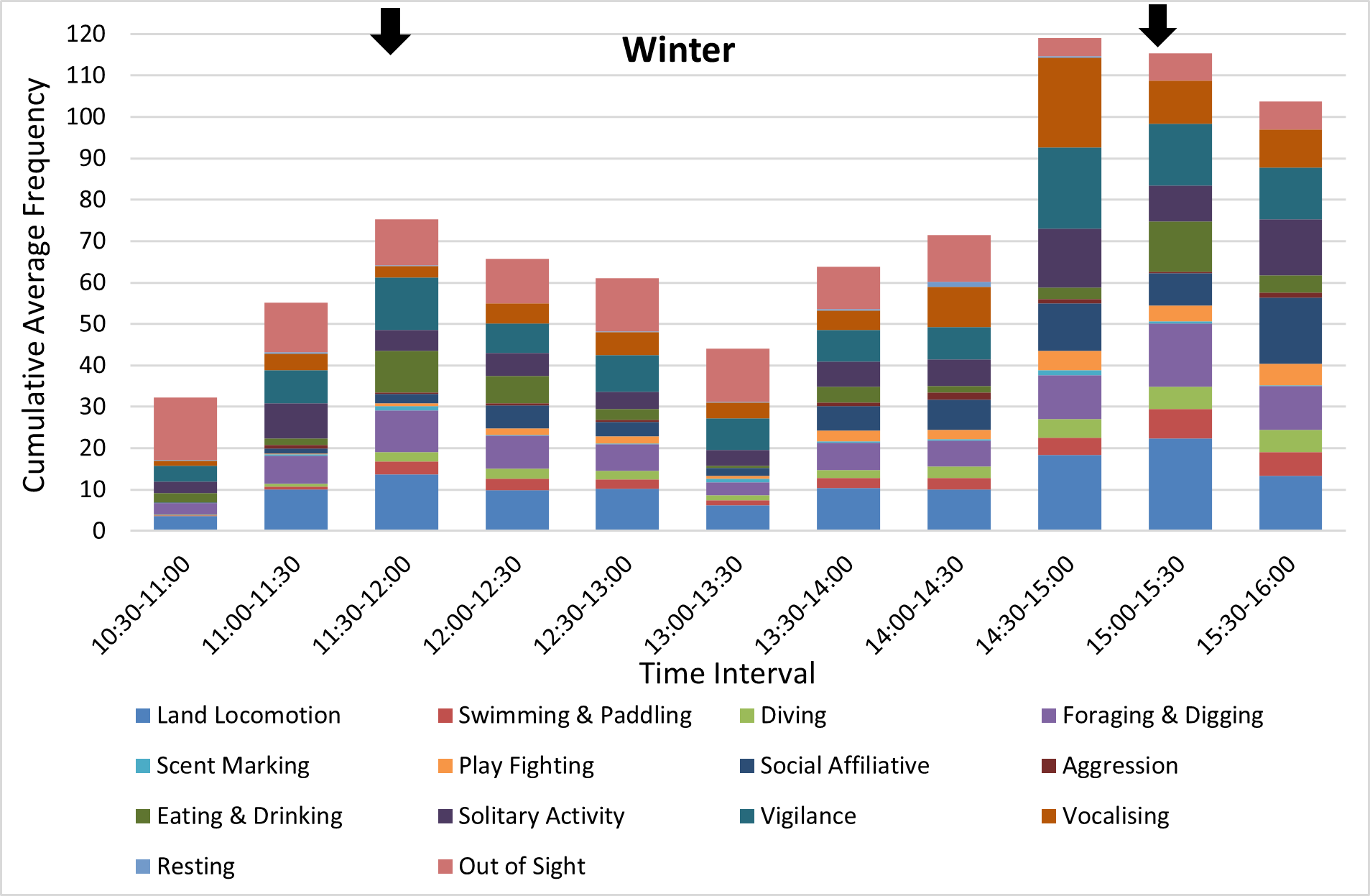
The peaks in otter activity around the two feeding times that occurred during the data collection intervals (the second feed of the day at 11:30 am and the third at 3:00 pm) were also associated with peaks in visitor numbers (Figure 11).
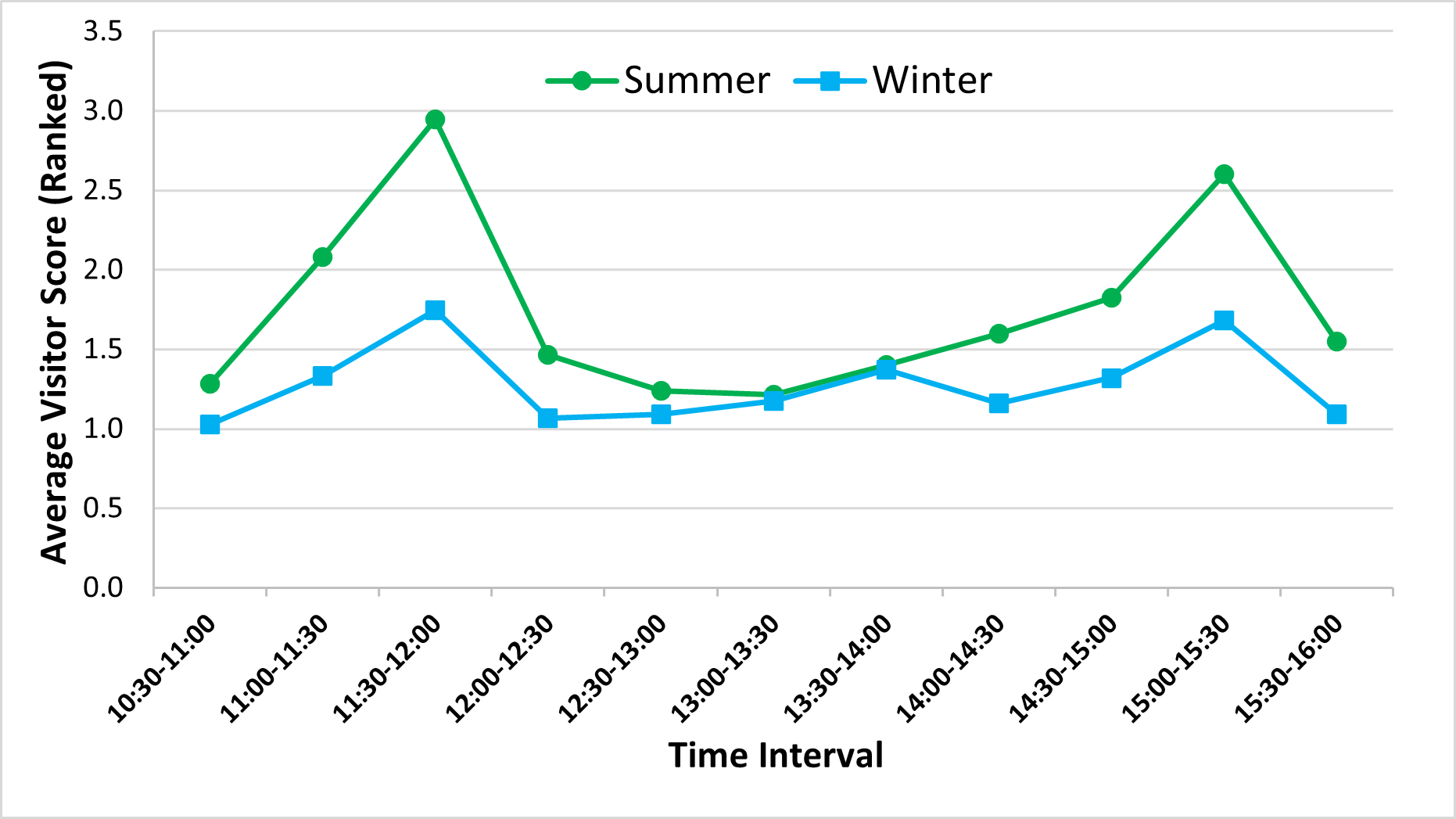
This pattern was more consistent in the summer, when all otters were inside the den for around one whole hour, which included the 1:00-1:30pm interval on all five days of data collection, with the exception of one instance of social affiliative behaviour recorded for the adults. In winter, the timing and the duration of the period of rest between the feeds was more variable, with one or both adult otters being out of sight inside the den more frequently than in the summer throughout the day, and with the otters at their most active later in the day, around the 3:00pm feed (Figure 10).
Cub Development
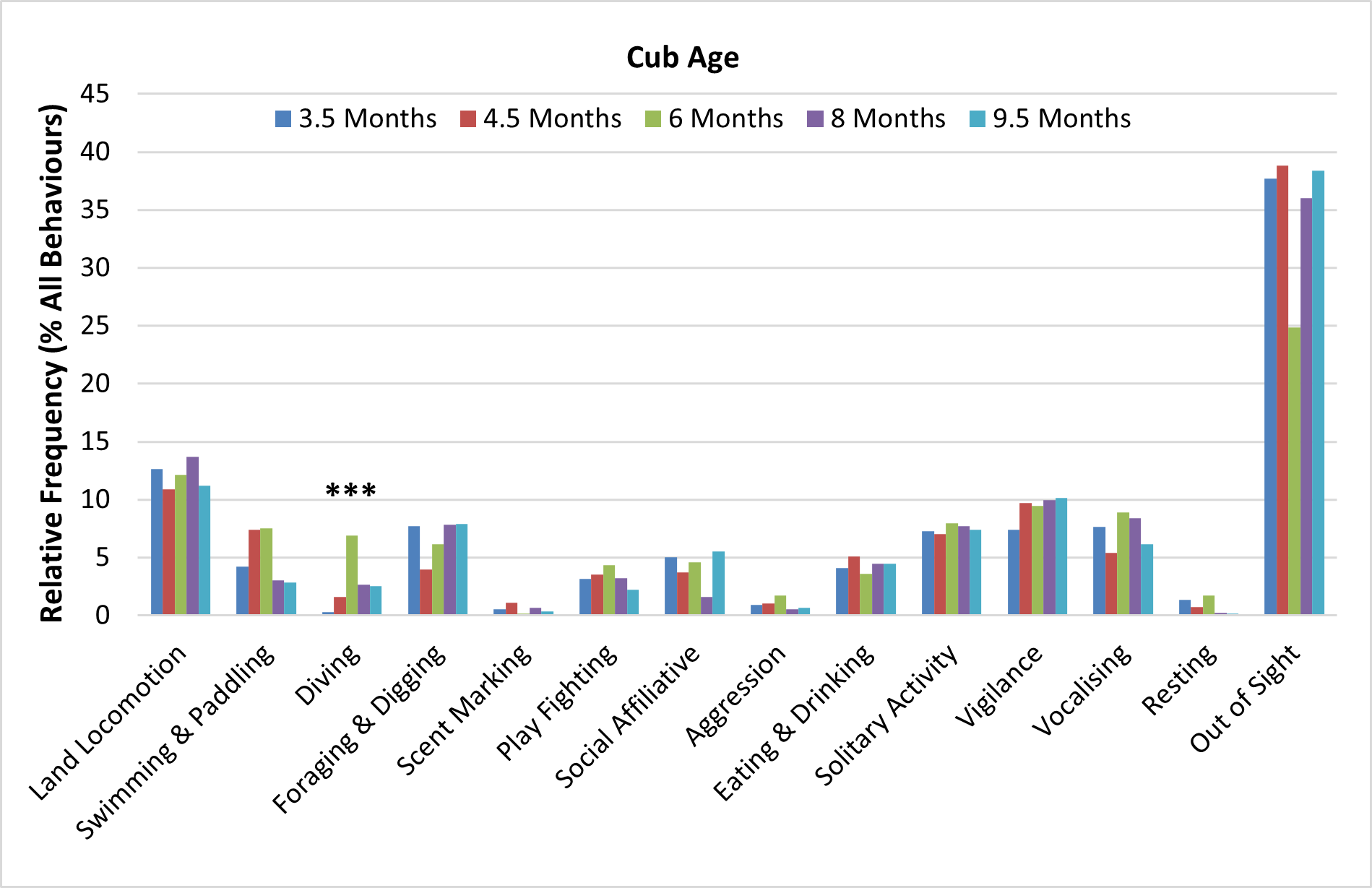
All four cubs born in March 2016 at WWT Washington progressed very well. By 4 weeks-old, they had roughly doubled their weight, compared to the first weigh-in at nearly 2 weeks-old (Figure 12, Table 3) and were seen outside from 2 months-old. By 3.5 months old (June 2016), although they had not been weaned yet, they were spending more time outside around feeding times, eating solid food and displaying a much greater variety of behaviours (Figures 13-14, Table 3). The relative frequency of swimming & paddling and diving increased from 3.5 months-old to 6 months-old, with the relative frequency of diving at 6 months-old being significantly higher than at all other stages (6.9% > [0.3-2.7%]; Z=28.588; p<0.001) (Figure 14). The relative frequency of cubs being ‘out of sight’ was at its lowest in August 2016, at 6 months-old.
Figure 12. Early photographic records of the otter cubs born in March 2016 (a-c) (Photos Copyright a-c: WWT) and of early swimming lessons (d) (Photo Copyright: Jans Bartholomew)
| Table 3 : Summary of otter cubs development at WWT Washington in 2016 (based on direct observations and information from WWT Washington). | ||||
| Age | Notes | |||
| 12 days (week 2) | First weigh in: 115-160g | |||
| 26 days | Second weigh in: 295-325g | |||
| 59 days 2 months-old | Cubs already taking short trips outside after feeding, especially in good weather. | |||
| 66 days 2 months-old | Cubs coming out on their own at feeding time; eating prawns; squeaking; climbing on top of each other to get back into the den. | |||
| 103 -110 days 3.5 months-old | Eating larger pieces of solid food, foraging close to parent or older sibling; moving around as a group with parent/ older sibling at other times; swimming with head out, climbing on the lower tree stumps; seen suckling outside. | |||
| 118 days 4 months-old | Playing, juggling pebble, rolling on grass or bark, vigilance standing, still moving around the enclosure mostly as a family group. Ash seen climbing on the tallest tree stump. (Data grouped with those for 3.5 months-old in Figure 14) | |||
| 139 days 4.5 months-old | More independent, occasionally seen foraging or playing further away from parents, swimming more in the deeper pool and occasionally with head under the water (recorded as diving). | |||
| 181 days 6 months-old | Even more independent, not following adults as much, more solitary activities (eating, juggling), lots of play fighting (water), swimming and diving in the deep pool. | |||
| 8-9.5 months-old | Very independent, doing things both on their own and as a family group; lots of foraging, diving, climbing, digging, running, exploring, play fighting and playing with pebbles twigs, straw, eggs, shells; adults still very vigilant and responsive to strange noises. | |||
Figure 13. Behaviours displayed by otter cubs at WWT Washington in June 2016: a) Suckling and resting close to adult female; b) Vigilance standing; c) Juggling round object (avocado seed). (Photos Copyright a-c: Mirela Cuculescu-Santana)
Mating Behaviour
Mating behaviour of the adult pair was observed several times on the days of data collection, in both seasons (Table 4), around midday and in early afternoon, only in the water in summer and only out of water in winter. The duration varied in both seasons, from 1 minute (nudging, clasping, gentle biting) to 14-16 minutes (holding on to each other in the water and rolling around and splashing together, in summer, or mounting on the grass, holding on to one another, rolling together, moving against each other, nudging and gentle biting in winter).
The video recording of one of the longer encounters that took place on the grass in the front area of the enclosure in November 2016 is available as supplementary material (ASCO Mating WWT Washington November 2016). Mating started with the male holding on to the female and moving rapidly against her in one of the positions shown in Figure 15 (a-c) in what appeared to be genital stimulation without copulation. This was followed by the female mounting the male (Figure 15d) and moving slower against him, when actual copulation took place.
Figure 15. Adult otters mating out of water in November 2016: a) male mounting the female; b)-c) male rubbing against female, with no copulation; d) female mounting the male and copulation with slower movements. (Photos Copyright a-d: Mirela Cuculescu-Santana; Video available as additional material at ASCO Mating WWT Washington November 2016 ; Video Copyright: Mirela Cuculescu-Santana)
DISCUSSION
Seasonal and Enrichment Influence
The Asian small-clawed otters (ASCO) at the WWT Washington were active outdoors in both summer and winter 2016, with peaks of activity and more diverse behaviours displayed around feeding times. The feeding strategy used included four feeds a day, with food scattering and hiding around the enclosure. The outdoor area had a variety of structural enrichments (natural substrates, vegetation, water features and climbing structures) in very good compliance with the guidelines for the species (AZA, 2009). The otters displayed several naturalistic behaviours, such as foraging on land and in water, digging, paddling, swimming, diving and social play, similar to those described for otters in the wild (Kruuk, 2006, Hussain et al., 2011) and associated with high welfare in captive otters (AZA, 2009).
As expected, there were significant seasonal differences in the behaviour of both adults and cubs, correlated with significant differences in enclosure use. The adult pair and the four cubs born in March 2016 swam (adults 7.1% summer > 2.9% winter; cubs 5.5% summer > 2.9% winter) and interacted with the water features significantly more frequently during the summer (adults 24% summer > 14% winter; cubs 19% summer > 14% winter), when the water was warmer (15-25 °C; average 19 °C; n=5 days). The adults also dived significantly more frequently in summer (5% > 2.6% winter), while for the cubs the relative frequency of diving was significantly higher in winter (2.6% > 1.9% summer), when they were overall more confident swimmers and divers.
In summer the pools were used for paddling, swimming, diving, foraging, play-fighting and sexual behaviour (the adults). Adults and cubs were often seen diving or searching the surrounding vegetation for scattered or hidden food, then eating it while still in shallow water. In winter, when the water was much colder (range 3.5-8 °C; average 6.5 °C; n=5 days), the otters still used the pools for swimming, diving, foraging and play-fighting, but less frequently than in the summer. Several occurrences of 4 minutes or longer spent continuously in the water were recorded for both the focal adult (the male) and cub (the white-furred female cub) in summer, while none were recorded in winter, when the majority of the swimming bouts lasted 1 minute or less. The adults used the den significantly less frequently in summer compared to winter and both adults and cubs were seen resting outside significantly more frequently in summer than in winter.
Similar findings were reported for ASCO living in a non-climatised indoor enclosure, who spent 33.4% of their time swimming in the summer, in water at 18-19 °C, compared to only 14.1% in winter, in water at 11-12 °C, when they spent more time resting and displayed more frequent begging and aggression (Cuculescu-Santana et al., 2017). Continuous swimming for more than 1 minute was never recorded in winter in the indoor pool and the longest swimming bout recorded in summer lasted 3 minutes (Cuculescu-Santana et al., 2017), when even in the summer the water was rarely warmer than 18.3 °C, the lowest recommended water temperatures for this species (AZA, 2009). As tropical warm-adapted mammals, ASCO have higher metabolic costs for thermoregulation than other Mustelids, especially in water below 16°C, which is estimated to be close to the lower extreme of their thermoneutral zone (Borgwardt and Culik, 1999). The metabolic rate of ASCO during rest on land at 16°C almost doubled when resting in cold water at 12 °C and increased further during swimming and foraging (Borgwardt and Culik, 1999). Movement in cold water increases the heat loss through forced convection through the skin (Hind and Gurney, 1997), which in ASCO is not fully compensated by the heat generated in muscles (Humphries and Careau, 2011). ASCO and another tropical species, the giant otter Pteronura brasiliensis, have shorter primary hairs in their coat than other otter species and thermal imaging showed they lost heat through the entire body surface, including the tail, while the better cold-adapted Eurasian river otter (EARO) Lutra lutra dissipated heat mainly through their feet (Kuhn, 2009; Kuhn and Meyer, 2009). EARO increase their body temperature through more activity on land before a foraging dive in cold water, which allows them to remain in the water until their body temperature decreases again to their average resting value (Kruuk et al., 1994a; Kruuk et al., 1997).
The structural enrichments added in autumn to some of the back and lower end areas of the otter enclosure at WWT Washington increased significantly the frequency of use of these areas in winter, probably due to initial novelty factor as well as longer-term increase in the spatial complexity of the ground areas and very likely contributed to maintaining good levels of diurnal activity and high display value in the cold season. The cubs played for up to 20-30 minutes at a time inside and around the newly added nest box and small holt and on the logs at the back of the enclosure, after the vegetation around them was trimmed. Frequent changes in enclosure “furniture” and management of the natural vegetation are among the recommended practices to stimulate active exploration (AZA, 2009) and enhance the quality of the space for captive otters (Foster-Turley et al., 1990). The front area of the enclosure was used significantly less frequently in winter compared to summer, when it was used for access to the pools, drying after swims and for resting outside and affiliative social interaction.
The levels of aggression were low in both seasons (0.6-1%). Vigilance (8-12%) and vocalisations (7-8%) were among the most frequently recorded active behaviours in both seasons, suggesting that the adults were very alert due to the presence of the cubs and used contact calls to co-ordinate the activities of the family group, such as swimming lessons or foraging. Soft calls (chirps) could be heard every time the family group was on the move, and often during periods of low activity, too, similar to the contact vocalisations described by Lemasson et al. (2014). The roles within the family group at WWT Washington were also similar to those described by Lemasson et al. (2014) for a family of ASCO at a zoo in France, with the adult male leading the group and the adult female carefully watching the surroundings during movement to the pools and back to their resting area. The male and the 1-year old cub were play-fighting more with the cubs and the female was usually surrounded by cubs during social rest. The male and the female were often seen resting together outdoors, in close body contact. As expected, the cubs displayed more play-fighting than the adults and less allogrooming and body contact. Play-fighting is a complex behaviour that is essential for the development of social as well as cognitive and emotional competencies in juvenile mammals (Pellis and Pellis, 2013; Palagi et al, 2016), and was another indicator of high welfare as it was often seen in wild otters (Kruuk, 2006; Reed-Smith et al., 2014).
The activity patterns observed in both summer and winter were consistent with the existence of a rhythm of activity entrained by fixed feeding times (Mistlberger, 2011; Carneiro and Araujo, 2012) and with those reported for ASCO at other establishments (Hawke et al., 2000; Ross, 2002; Cuculescu-Santana et al., 2017). The higher levels of activity around feeds were related to appetitive behaviours indicative of good welfare (Watters, 2014) and were paralleled by peaks in visitor numbers. Several visitors also stayed to watch the otters during the quieter periods of social play, solitary play or rest between feeds. The frequency of repetitive behaviours associated with more stressful feeding anticipation, such as vigilance standing and loud short calls (Scheifele et al., 2015) was higher in winter, especially before the 3:00pm feed, suggesting greater hunger due to the increased energetic costs of thermoregulation in the cold season (Gothard, 2007; Cuculescu-Santana et al., 2017). At other times, vigilance standing was usually displayed in response to unfamiliar noises or events (eg a maintenance vehicle stopping next to their enclosure), rather than as repetitive “begging” to keepers or visitors. Peaks in activity associated with food acquisition have been described for wild otters (Hussain, 2013). Their timing appeared to be influenced by seasonal temperatures (Reed-Smith et al., 2014) and by the patterns of nearby human activity (Foster-Turley, 1992; Castro and Dolorosa, 2006).
Parental Care and Reproductive Behaviour
The adult pair displayed more social affiliative behaviours in the summer, associated with more parental care (e.g. suckling, food sharing, cub grooming, play) and with periods of lower levels of activity outside, when social grooming and body rubbing between the adults were recorded, while the cubs were still inside the den. The pair displayed sexual behaviour in both seasons, for up to 14-16 minutes, usually in the early afternoon, at quieter times. Giant otters at a zoo in Colombia also displayed sexual behaviour at times with fewer visitors around (Corredor-Londono and Tigreros-Munoz, 2006). Courtship behaviours in ASCO include genital stimulation and the female mounting the male (Kuenzer and Lombardi, 1998) and the out-of-water sexual interaction observed at WWT Washington appeared to involve first a stage of stimulation during which the male was rubbing against the female, without copulation, in ventral-to-dorsal or ventral-to-ventral position, followed by copulation during which the female mounted the male in a ventral-to-ventral position and moved slower against him.
ASCO are reproductively active all year round (Bateman et al., 2009) and the fact that mating at WWT Washington was observed only in the water in summer and only out of water in winter, when the water temperature was below 10 °C on all days of data collection, suggested that ASCO adjusted their sexual behaviour to compensate for the increased cost of thermoregulation in cold water (Borgwardt and Culik, 1999). ASCO is among the otter species whose behaviour is least studied in the wild and most of the information about mating behaviour is based on observations in zoos outside its natural distribution range (Hussain et al., 2011). Copulation can last up to 45 minutes and can occur several times a day, both on land and in water, with the dorsal-ventral position being the most common (Reed-Smith and Polechla, 2002). In giant otters and sea otters the male bites the female during mating and copulation may last up to 20-30 minutes (Kruuk, 2006). Only gentle biting occurred at WWT Washington during mating out of water, but the instances of sexual behaviour that took place in the water during the summer involved more energetic rolling around, tumbling, lots of splashing, vocalisations and possibly more aggressive biting, too. EARO seen mating in the water in spring in Shetland also had a quieter period of holding on to each other after rolling, tumbling and splashing around (Kruuk, 2006).
ASCO produce litters of 1-7 cubs, with an approximate sex ratio of 1:1 (Hussain et al, 2011). The pair at WWT Washington produced three successful litters: one female cub (2015), the four included in this study (3:1; 2016) and four more (2:2) in 2017. Both parents were involved in caring for the cubs, with help from the 1-year old sibling, as reported for other captive ASCO pairs (Sivasothi, 1998; Nair and Agoramoorthy, 2002; Hussain et al, 2011).
Cub Development
The cubs at WWT Washington were already paddling and swimming with the head out of water around 3.5-4 months-old (15-17 weeks), always accompanied by the adults, with frequent contact calls, but were only rarely seen with the head under water (diving). Their development was consistent with other reports for captive ASCO up to weaning age (AZA, 2009; Hussain et al., 2011). At 16 weeks-old they were not completely weaned, as suckling was observed during periods of rest outside. ASCO cubs looked after by their parents usually begin to come out of the birthing den around 2 months-old, eat first solids and begin swimming lessons with parents around 2-3 months-old and are weaned around 3.5-months old (Hussain et al., 2011). Leslie (1971) described 3 months-old ASCO cubs playing outside with their parents, exploring all areas of the enclosure apart from the pool (the parents disliked the water) and starting to enjoy the water after being pushed in by keepers, even taking turns at repeatedly sliding down the embankment into the water. Two hand-reared ASCO cubs at Miami Metro Zoo showed interest in solid food and were able to catch small fish at 8 weeks-old, started playing in shallow water around 2 months-old, were swimming in a deep pool by around 3.5 months-old and were weaned around 4.5 months-old (Webb, 2011).
By 4-4.5 months-old the cubs at WWT Washington were displaying several otter-specific behaviours. They climbed confidently on the structures in the enclosure, were rolling on the grass or bark to dry their fur after swimming and displayed vigilance standing, which is a sign of alertness to unfamiliar noises and possible danger in wild otters (Kruuk, 2006). They also juggled pebbles, demonstrating forepaw dexterity (Larivière, 2003) which is particularly important for ASCO as hand-oriented predators (Timm-Davis et al., 2015). Wild ASCO capture most of their food by searching with their forepaws under rocks and in crevices on the bottom of water bodies, which in captivity is reflected in well-developed forms of object manipulation and play (Pellis, 1984) and ability to open different types of containers and solve puzzles to extract food (Ladds et al., 2017; Allison et al., 2020).
The relative frequency of swimming and paddling increased from 3.5 to 4.5 months-old, while the relative frequency of diving was still low at 4.5 months-old and increased significantly only around 6 months-old, as the cubs became more confident swimmers. The lowest frequency of being inside the den was recorded at 6 months-old (end of August 2016), when the cubs spent a lot of time outside, moving around the enclosure, swimming and play-fighting, and demonstrating an increased level of independence, especially when foraging around feeding times. In the wild ASCO live in social groups but forage individually (Kruuk et al., 1994b). By 8-9.5 months-old the cubs were even more independent and their behavioural budget was similar to that of the adults, apart from sexual behaviour. They were already confident swimmers and divers and were often seen at some distance from the adults, retrieving successfully pieces of food from the pools, suggesting competence with both diving and aquatic foraging skills by this age.
The ability to acquire enough food around 14 weeks-old, when weaning usually occurs appeared critical for the survival of cubs (Prima, 1992) and many captive cub deaths occurred around this age. There is very little information in the peer-reviewed literature about ASCO cub development post-weaning and it is not known how they develop in the wild (Kruuk, 2006). There are reports of sightings of family groups (Foster-Turley et al., 1990; Sivasothi and Nor, 1994; Castro and Dolorosa, 2006) and of adults carrying cubs with their forepaws or under their chins (Sivasothi and Nor, 1994). In species with complex group structure, such as giant otters and ASCO, cubs remain dependent on mothers or on family group for long periods of time until they develop adequate food acquisition skills (Kruuk, 2006). For captive ASCO it is recommended that cubs remain with their parents up to around one year-old, to have the chance to learn not only survival skills, but also how to raise cubs (Heap et al., 2008). Yearling ASCO cubs at Chester Zoo spent more time in solitary behaviours such as digging and playing with objects and less time engaged in social interaction than the adults (Owen, 2004).
Behavioural ontogeny in other species of otter follows similar patterns. In sea otters the period of cubs dependence on the mother varies with geographical area, from 6 to 12 months-old, longer where the predominant food is fish (Kruuk, 2006). The period of dependence is usually longer in other species for which the main food is fish, such as giant otters (GO) and North American (NARO) and Eurasian river otters (EARO). GO have a complex social organisation, similar to that of ASCO, maintained by complex vocalisations and frequent mutual grooming (Mumm and Knornschild, 2014; Groenendijk, 2019). GO cubs travel and catch fish with their family group from 3-4 months-old, often play in the water while the adults are resting out of water, and are protected and fed cooperatively by all members of the group (Evangelista and Rosas, 2011). They are completely weaned by 5-6 months and stay with the family group up to 2 years-old (Kruuk, 2006). Eurasian otter cubs (EARO) begin weaning around 2 months-old and start fishing as a family group with their mother from 8-10 weeks-old, with frequent contact calls (Kruuk, 2006). Fishing lessons and prey capture play were seen in wild EARO and although by 4-5 months-old the cubs were able to catch fish, they remained with the family up to 10-11 months-old or even longer, presumably because underwater hunting skills take longer to develop and require more practice than terrestrial hunting (Kruuk, 2006). Captive EARO cubs were displaying uncoordinated surface swimming and diving from around 3 months-old, learning swimming and hunting techniques from their mother (Polotti et al., 1995). Similar to the ASCO cubs in our study, by 8 months-old they were spending less time with their mother, hunting and sprainting independently, and by 9 months-old they had developed coordinated surface swimming (Polotti et al., 1995). NARO cubs begin swimming around 2.5 months-old, take first deep dives and begin to fish at 3 months, become efficient and dextrous swimmers by 6 months-old, but can take up to 10 months-old to become efficient foragers and stay with their families up to 8-12 months-old (Shannon, 1998).
LIMITATIONS OF THE STUDY
We presented a case study based on observations carried out over 7 months at a single establishment, outlining the main changes in the behaviour and enclosure use of a family of Asian small-clawed otters under the combined influence of seasonality, cub age progression and on-going enrichment strategies, making it difficult to propose direct cause and effect relationships, as well as to replicate such a combination of conditions elsewhere.
CONCLUSIONS
- Seasonal temperatures had a significant influence on the behaviour and outdoor enclosure use of Aonyx cinereus at WWT Washington, who rested outside and used the pools significantly more frequently in summer, when the water temperature was closer to or within the 18.3-29.4 °C range recommended for the species, compared to winter, when the water temperatures were below 10 °C.
- The behavioural budgets of ASCO cubs from 3.5 to 9.5 months-old showed that the cubs were displaying vigilance, co-ordinated swimming and forepaw dexterity at 4.5 months-old and confident diving and underwater swimming at 6 months-old. By 9.5 months-old they were foraging independently, engaged more in social grooming, but still played more frequently than the adults.
- The feeding and structural enrichment strategies used at WWT Washington maintained good levels of activity outdoors and high display value all year round and very likely contributed to the successful reproduction of the ASCO pair, who produced a total of 9 cubs, some of which have already reproduced successfully themselves at the establishments where they were moved, emphasizing that captive ASCO need various forms of enrichment to stimulate behaviours similar to those in the wild and maintain good welfare all year round.
Acknowledgements: The authors would like to thank Gill Pipes (Centre Manager) and all staff at the WWT Centre Washington for their enthusiasm and support with this project and for looking after the family of otters. We would also like to thank Samantha Jameson for help with proof-reading the manuscript. The work was carried out within the standard workloads of staff and students at Northumbria University and staff at the WWT Washington.
Note - A shorter version of this work was presented at the BIAZA Research Conference, 4-5 July 2017, Edinburgh, Scotland, United Kingdom.
REFERENCES
Allison, M.-L., Reed, R., Michels, E., Boogert, N.J. (2020). The drivers and functions of rock juggling in otters. R. Soc. Open Sci. 7: 200141.
AZA (2009). Otter (Lutrinae) care manual. Silver Spring, MD: Association of Zoos and Aquariums. 154 pp.
Bateman, H.L., Bond, J.B., Campbell, M., Barrie, M., Riggs, G., Snyder, B., Swanson, W.F. (2009). Characterization of basal seminal traits and reproductive endocrine profiles in North American river otters and Asian small-clawed otters. Zoo Biol. 28: 107-126.
Borgwardt, N., Culik, B.M. (1999). Asian small-clawed otters (Amblonyx cinerea): resting and swimming metabolic rates. J. Comp. Physiol. B 169: 100-106.
Carneiro, B.T.S., Araujo, J.F. (2012). Food entrainment: Major and recent findings. Front. Behav. Neurosci. 6: 83. doi: 10.3389/fnbeh.2012.00083.
Castro, L.S.G., Dolorosa, R.G. (2006). Conservation status of the Asian small-clawed otter Amblonyx cinereus (Illiger, 1815) in Palawan, Philippines. Philipp. Sci. 43: 69-76.
Cianfrani, C., Broennimann, O., Loy, A., Guisan, A. (2018). More than range exposure: Global otter vulnerability to climate change. Biol. Conserv. 221: 103-113.
CITES (2019) Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III (26/11/2019). United Nations Environment Programme, 75p.
Corredor Londono, G., Tigreros Munoz, N. (2006). Reproduction, behaviour and biology of the Giant river otter Pteronura brasiliensis at Cali Zoo. Int. Zoo Yb. 40: 360-371.
Cuculescu-Santana, M., Horn, C., Briggs, R.N., Bowe, C., Geraughty, M.L. (2017). Seasonal changes in the behaviour and enclosure use of captive Asian small clawed otters Aonyx cinereus. IUCN Otter Spec. Group Bull. 34(1): 29-50.
Dornbusch, T., Greven, H. (2009). Notes on keeping of Asian small-clawed otters (Amblonyx cinereus) in European zoos. Zool. Garten 78: 75-80. [In German with English abstract].
Evangelista, E., Rosas, F.C.W. (2011). Breeding behavior of giant otter (Pteronura brasiliensis) in the Xixuau Reserve, Roraima, Brazil. IUCN Otter Spec. Group Bull. 28(A): 5-10.
Foster-Turley, P. (1986). A Progress Report on the Species Survival Plan for Asian Small Clawed Otters in United States Zoos. IUCN Otter Spec. Group Bull. 1: 19 – 21.
Foster-Turley, P. (1990). Otters in captivity. In: Foster-Turley, P., Macdonald, S., Mason, C. (eds.) Otters. An action plan for their conservation. Proceedings of the IUCN Otter Specialist Group Meeting, Gland, Switzerland. p 17-21.
Foster-Turley, P. (1992). Conservation aspects of the ecology of Asian small-clawed and smooth otters on the Malay Peninsula. IUCN Otter Spec. Group Bull. 7: 26-29.
Foster-Turley, P., Macdonald, S., Mason, C. (1990). Otters. An action plan for their conservation. Proceedings of the IUCN Otter Specialist Group Meeting, Gland, Switzerland. 133 p.
Gothard, N. (2007). What is the proximate cause of begging behaviour in a group of captive Asian short-clawed otters? IUCN Otter Spec. Group Bull. 24: 14-35.
Groenendijk, J. (2019). The Giant Otter: Giants of the Amazon. Pen & Sword Books Ltd. Yorkshire – Philadelphia. 214 p.
Harrington, L.A., Macdonald, D.W., D’Cruze, N. (2019). Popularity of pet otters on YouTube: evidence of an emerging trade threat. Nat. Conserv. 36: 17–45.
Hawke, L., Lauer, P., Bartholomeusz, D., Steen, Z. (2000). Effects of increased food dispersal and random feeding time/ place on stereotyped behaviours in otters at Adelaide Zoo. Int. Zoo News 47: 71-81.
Heap, C.J., Wright, L., Andrews, L. (2008). Summary of husbandry guidelines for Asian small clawed otters in captivity. IUCN/ SSC Otter Specialist Group, Otters in Captivity Task Force. 17 p.
Hind, A.T., Gurney, W.S.C. (1997). The metabolic cost of swimming in marine homeotherms. J. Exp. Biol. 200: 531-542.
Humphries, M.M., Careau, V. (2011). Heat for nothing or activity for free? Evidence and implications of activity-thermoregulatory heat substitution. Integr. Comp. Biol. 51: 419-431.
Hussain, S.A. (2013). Activity pattern, behavioural activity and interspecific interaction of smooth coated otter (Lutrogale perspicillata) in National Chambal Sanctuary, India. IUCN Otter Spec. Group Bull. 30: 5-17.
Hussain, S.A., Gupta, S.K., de Silva, P.K. (2011). Biology and ecology of Asian small-clawed otter Aonyx cinereus (Illiger, 1815): A review. IUCN Otter Spec. Group Bull. 28: 63-75.
Kitade, T., Naruse, Y. (2018). Otter Alert: A rapid assessment of illegal trade and booming demand in Japan. TRAFFIC Japan Office, 31 p.
Kruuk, H. (2006). Otters: ecology, behaviour and conservation. Oxford University Press. 265 p.
Kruuk, H., Balharry, E., Taylor, P.T. (1994a). Oxygen consumption of the Eurasian otter Lutra lutra in relation to water temperature. Physiol. Zool. 67: 1174-1185.
Kruuk, H., Kanchanasaka, B., O’Sullivan, S., Wanghongsa, S. (1994b). Niche separation in three sympatric otters, Lutra perspicillata, L. lutra and Aonyx cinerea in Huay Kha Khaeng, Thailand. Biol. Conserv. 69: 115-120.
Kruuk, H., Taylor, P.T., Mom, G.A.T. (1997). Body temperature and foraging behaviour of the Eurasian otter (Lutra lutra), in relation to water temperature. J. Zool. 241: 689-697.
Kuenzer, D., Lombardi, D. (1998). Behavior and Social Organization. In: Lombardi, D., O’Connor, J. (eds). Asian small-clawed otter (Aonyx cinerea): Husbandry manual. American Zoo and Aquarium Association. p 61-63.
Kuhn, R. (2009). Comparative analysis of structural and functional hair coat characteristics, including heat loss regulation, in the Lutrinae (Carnivora: Mustelidae). PhD Thesis. University of Hamburg, Hamburg, Germany. 225 pp.
Kuhn, R.A., Meyer, W. (2009). Infrared thermography of the body surface in the Eurasian otter Lutra lutra and the giant otter Pteronura brasiliensis. Aquat. Biol. 6: 143-152.
Ladds, Z., Hoppitt, W., Boogert, N.J. (2017). Social learning in otters. R. Soc. open sci. 4: 170489.
Lancaster, W.E. (1975). Exhibiting and breeding the Asian small-clawed otter. Int. Zoo Yb. 15: 63-65.
Larivière, S. (2003). Amblonyx cinereus. Mamm. Species 720: 1-5.
Lemasson, A., Mikus, M.-A., Blois-Heulin, C., Lode, T. (2014). Vocal repertoire, individual acoustic distinctiveness, and social networks in a group of captive Asian small-clawed otters (Aonyx cinerea). J. Mammal. 95: 128-139.
Leslie, G. (1971). Further observations on the Oriental short-clawed otter Amblonyx cinerea at Aberdeen Zoo. Int. Zoo Yb. 11: 112-113.
Mistlberger, R.E. (2011). Neurobiology of food anticipatory circadian rhythms. Physiol. and Behav. 104: 535-545.
Mumm, C.A.S., Knornschild, M. (2014). The vocal repertoire of adult and neonate Giant Otters (Pteronura brasiliensis). PLoS ONE 9(11): e112562. doi:10. 1371/journal.pone.0112562.
Nair, S., Agoramoorthy, G. (2002). Mating- and birth-related behaviour in captive Asian small clawed otters. Int. Zoo News 49(1): No. 314. 4 p.
Owen, C. (2004). Do visitors affect the Asian short-clawed otter Aonyx cinerea in a captive environment? 6th Annual Symposium on Zoo Research. BIAZA. p 202-211.
Palagi, E., Burghardt, G.M., Smuts, B., Cordoni, G., Dall’Olio, S., Fouts, H.N., Rehakova-Petru, M., Siviy, S.M., Pellis, S.M. (2016). Rough-and-tumble play as a window on animal communication. Biol. Rev. 91: 311-327.
Pellis, S.M., Pellis, V.C. (2013). Rough-and-tumble play and the development of the social brain. Curr. Dir. Psychol. Sci. 16: 95-98.
Pellis, S.M. (1984). Two aspects of play-fighting in a captive group of Oriental small-clawed otters Amblonyx cinerea. Z. Tierpsychol. 65: 77-83.
Polotti, P., Prigioni, C., Fumagalli, R. (1995). Preliminary data on the ontogeny of some behavioural activities of captive otter cubs. Hystrix, 7(1-2): 279-284.
Prima, C. (1992). Weaning behaviour in the Oriental small-clawed otter Aonyx cinerea at the National Zoological Park, Washington, DC. Int. Zoo Yb. 31: 245-250.
Reed-Smith, J. (1998). Reproduction. In: Lombardi, D., O’Connor, J. (eds). Asian small-clawed otter (Aonyx cinerea): Husbandry manual. American Zoo and Aquarium Association. p 49-52.
Reed-Smith, J., Polechla, P.J.Jr. (2002). Husbandry and captive breeding of otters (Lutrinae) in North and Latin America: A synopsis of current progress. Proc. VIIth International Otter Colloquium, p 276-281.
Reed-Smith, J., Serfass, T., Samweli Kihudu, T., Mussa, M. (2014). Preliminary report on the behaviour of spotted-necked otter (Lutra maculicollis, Lichtenstein, 1835) living in a lentic ecosystem. Zoo Biol. 33: 121-130.
Rees, P.A. (2015). Studying captive animals: A workbook of methods in behaviour, welfare and ecology. Wiley-Blackwell. 301 p.
Rosli, M.K.A., Syed-Shabthar, S.M.F., Abdul-Patah, P., Abdul-Samad, Z., Abdul, S.N., Burhanuddin, M.N., Zulkifli, N.A., Shukor, M.N., Budsabong, K., Changtragoon, S., Sekiguchi, T., Sasaki, H., Md-Zain, B.M. (2014). A new subspecies identification and population study of the Asian small-clawed otter (Aonyx cinereus) in Malay Peninsula and Southern Thailand based on fecal DNA method. The Scientific World J. 2014: 457350.
Ross, S.R. (2002). The effect of a simple feeding enrichment strategy on the behaviour of two Asian small-clawed otters (Aonyx cinerea). Aquat. Mammals 28: 113-120.
Scheifele, P.M., Johnson, M.T., Fry, M., Hamel, B., Laclede, K. (2015). Vocal classification of vocalizations of a pair of Asian Small-Clawed otters to determine stress. J. Acoust. Soc. Am. 138: EL105-EL109.
Shannon, J.S. (1998) Behaviour of Otters in a Marine Coastal Habitat: Summary of a Work in Progress. IUCN Otter Spec. Group Bull. 15(2): 114 - 117
Sivasothi, N. (1998). Asian otters in captivity. Asian Otter Newsletter 5: 15-18.
Sivasothi, N., Nor, B.H.M. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia 285: 151-170.
Timm-Davis, L.L., DeWitt, T.J., Marshall, C.D. (2015) Divergent skull morphology supports two trophic specializations in otters (Lutrinae). PLoS ONE 10(12): e0143236.
Timmis, W.H. (1971). Observations on breeding the oriental short clawed otter Amblonyx cinerea at Chester Zoo. Int. Zoo Yb. 11: 109-111.
Washington Wetland Centre (2016). Wildfowl and Wetlands Trust Washington: What’s On? Available from: https://www.wwt.org.uk/wetland-centres/washington/whats-on/ . Accessed 1 May 2016.
Watters, J.V. (2014). Searching for behavioural indicators of welfare in zoos: Uncovering anticipatory behaviour. Zoo Biol. 33: 251-256.
Webb, T.D. (2011). Hand rearing 1.1. Asian small clawed otter (Amblonyx cinereus) 21 February 2007 through 2 July 2007. IUCN Otter Spec. Group Bull. 28(A): 108-113.
Wright, L.C. (2003). Assessing the welfare of captive Asian small-clawed otters (Amblonyx cinereus): Can inductive methods play a part? IUCN Otter Spec. Group Bull. 20: 35-41.
Wright, L.C. (2005). Interesting cooperative bedding gathering behaviour in captive Asian small clawed otters (Amblonyx cinerea). IUCN Otter Spec. Group Bull. 18(1): 25 – 28.
Wright, L., de Silva, P., Chan, B., Reza Lubis, I. (2015). Aonyx cinereus. The IUCN Red List of threatened species 2015. Downloaded 30 January 2016. Available from http://dx.doi.org/10.2305/IUCN.UK.20152.RLTS.T44166A21939068.en .
Résumé: Utilisation de l’Enclos Exterieur et Comportement chez l’aAdulte et le Loutron de la Loutre Cendrée Aonyx cinereus en Été et e0n Hiver
Le comportement et l’utilisation de l’enclos extérieur d'une famille de loutres Aonyx cinereus ont été étudiés en été et en hiver au Centre Wildfowl and Wetland Trust Washington, au Royaume-Uni. En été, la nage, le pataugeage (adultes et jeunes) et la plongée (adultes) ont été observés de manière significative plus fréquemment qu'en hiver, ce qui est en corrélation avec des fréquences d'utilisation du milieu aquatique nettement plus élevées. Pour les loutrons, la fréquence relative de plongée était significativement plus faible qu'en hiver, car les jeunes apprenaient encore à nager et à se nourrir sous l'eau. Les niveaux d'activité et la diversité des comportements étaient plus élevés aux heures de repas durant les deux saisons. Les loutrons nageaient déjà dans des eaux peu profondes à l'âge de 3,5 mois et dans des eaux plus profondes à 4,5 mois, principalement en groupe familial. À l'âge de 3,5 à 6 mois, ils étaient beaucoup plus souvent dans la catiche, donc moins visibles que les adultes et montraient davantage de jeux combatifs. À l'âge de 8 à 9,5 mois, ils se déplaçaient de manière autonome, en quête de nourriture ou en jouant et leur attitude comportementale était similaire à celle des adultes. La jonglerie avec un objet et la station debout avec vigilance sont apparues à partir d'environ 4 mois, lors du sevrage. L'apport de structures supplémentaires (souches, terrier, nichoir) au début de l'automne a augmenté la fréquence d'utilisation d’espaces au sol en hiver, lorsque la température de l'eau était inférieure à 10 °C. Les stratégies d'alimentation et l’augmentation des structures utilisées ont été efficaces pour maintenir les loutres actives à l'extérieur et garder leur attractivité élevée durant la saison froide (températures de l'air en été de 15 à 27 °C > en hiver de 3,5 à 10 °C), soulignant l'importance de l’apport des structures pour un bien-être animal satisfaisant.
Revenez au dessus
Resumen: so de un Recinto Exterior, y Comportamiento de un Adulto y una Cría de Nutria de Uñas Pequeñas Asiática Aonyx ciniereus en Verano e Invierno
Investigamos el comportamiento y el uso de un recinto exterior, por una familia de Aonyx cinereus, en verano e invierno en el Wildfowl and Wetland Trust Washington, Reino Unido. En verano, la natación y propulsión acuática (adultos y crías) y el buceo (adultos) fueron registrados significativamente con más frecuencia que en invierno, correlacionado con frecuencias significativamente más altas de uso de los dispositivos acuáticos. Para las crías, la frecuencia relativa de buceo fue significativamente más baja comparada con el invierno, ya que las crías estaban aún aprendiendo a nadar y alimentarse bajo el agua. Los niveles de actividad y la diversidad de comportamientos fueron más altos alrededor de los momentos de alimentación, en ambas estaciones. Las crías ya estaban nadando en aguas poco profundas a los 3.5 meses de edad, y en aguas más profundas a los 4.5 meses de edad, mayormente como grupo familiar. A los 3.5-6 meses de edad estaban ocultas en la madriguera significativamente con más frecuencia que los adultos, y desplegaban más juego-pelea. Para los 8-9.5 meses de edad se movían independientemente, alimentándose o jugando, y su presupuesto de comportamientos era similar al de los adultos. El malabarismo con objetos, y pararse en posición vigilante, se desplegaron a partir de unos 4 meses de edad, cuando también ocurrió el destete. La introducción de enriquecimiento estructural adicional (troncos, cuevas, caja-nido) a principios de otoño incrementó la frecuencia de uso de las áreas terrestres en invierno, cuando las temperaturas del agua estaban por debajo de los 10° C. Las estrategias de alimentación y de enriquecimiento estructural utilizadas fueron efectivas para mantener a las nutrias activas en esta situación de aire libre, y para mantener su alto valor de exhibición en la estación fría (las temperaturas diurnas del aire en verano 15-27 °C > invierno 3.5-10° C), enfatizando la importancia del enriquecimiento para un adecuado bienestar.
Vuelva a la tapa