IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 1 (January 2021)
Citation: Michalski F., Martins, C.B., Rheingantz, M.L. and Norris, D. (2021). New Scent Marking Behavior of Neotropical Otter (Lontra longicaudis) in the Eastern Brazilian Amazon. IUCN Otter Spec. Group Bull. 38 (1): 28 -37
New Scent Marking Behavior of Neotropical Otter (Lontra longicaudis) in the Eastern Brazilian Amazon.
Fernanda Michalski1, 2, 3*, Cassiano Bueno Martins1, 2, Marcelo Lopes Rheingantz4, and Darren Norris1, 2, 5
1Postgraduate Programme in Tropical Biodiversity, Federal University of Amapá, Macapá, Amapá, Brazil, 68903-419
2Ecology and Conservation of Amazonian Vertebrates Research Group, Federal University of Amapá, Macapá, Amapá, Brazil, 68903-419
3Pro-Carnivores Institute, Atibaia, São Paulo, Brazil, 12945-010
4Ecology Department, Federal University of Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil, 21941-902
5School of Environmental Sciences, Federal University of Amapá, Macapá, Amapá, Brazil, 68903-4190
* Corresponding Author: Email:: fmichalski@gmail.com
(Received 14th May 2020, accepted 6th August 2020)
Abstract: Scent marking behavior in mammals is related with both inter and intra-specific communication. Several otter species are known to communicate via scent marking, but a couple scent marking has not been documented in the Neotropical Otter (Lontra longicaudis). We obtained field observations of scent marking behavior in Neotropical Otters over two years using camera traps along waterways in the eastern Brazilian Amazon. Our results reveal the use of sandy substrates on islands and river margins for intra-specific communication between otters. Most records (62.5%) were from solitary adults. We document multiple independent records of adult otters digging to scent mark with urine and couple behavior of males urinating on top of female’s fresh urine in newly dug shallow craters. We also demonstrate behavioral plasticity of this species evidenced by camera traps recording terrestrial activity during both day and night. Our results contribute to improve the knowledge of the behavior of this otter species in the wild and can potentially be applied to improve ex-situ welfare of captive otters.
Keywords: Digging, Couple behavior, Intra-species behavior, Mammal, Carnivore, Mustelidae
INTRODUCTION
Most mammals are known to scent-mark with urine, feces and sometimes with glandular secretions (Ralls, 1971; Johnson, 1973; Thiessen and Rice, 1976). Within this context, carnivores deploy their excreta for diverse reasons, with the use of scent marking showing substantial intra-specific variation (MacDonald, 1980). Scent marking functions in carnivores are known to be related to affirm dominance (Gese and Ruff, 1997; Allen et al., 2017), defend food resources (Piñeiro and Barja, 2015), maintain territories (Smith et al., 1989; Roper et al., 1993), selecting and advertising for mates (Allen et al., 2015; 2016), among other inter- and intraspecific communications.
Like most mustelids otters use scent marking to communicate (Johnson, 1973; Kruuk, 1992; Ben-David et al., 2005; Kean et al., 2011). Scent marking in otters can occur in different forms, for example, mucus may be added to the spraint prior to deposition or mucus may occur in isolation without fecal material, which suggests a more complex cause and function that simply as a feces-finding substance (Kruuk, 2006; 2014). Several hypotheses for the functions of scent marking by Eurasian Otters (Lutra lutra) have already been tested, with results showing that spraints at latrines function to communicate social status of males (Rostain et al., 2004). Scent marking helps maintain the social system in cooperative species and has been shown to be important for group dynamics of territorial Giant Otters (Pteronura brasiliensis) (Carter and Rosas, 1997; Leuchtenberger and Mourão, 2009). The more solitary Neotropical Otters (Lontra longicaudis) also use scent marking for communication between individuals, with information transmitted by the deposition of spraint, feces and mucus in conspicuous locations such as rocks, fallen tree trunks, and sand banks along rivers (Dunstone and Strachan, 1988; Rheingantz et al., 2016; 2017; Roberts et al., 2016). This communication between Neotropical Otters has been linked with the role in determining space use and sexual behavior (Larivière, 1999).
Although the Neotropical Otter (Lontra longicaudis) is classified as Near Threatened by the IUCN and has a wide distribution in the Neotropics (Rheingantz and Trinca, 2015), there are several gaps in our knowledge of this species behavior (de Almeida and Ramos Pereira, 2017; Rheingantz et al., 2017). Indeed, due to the difficulty in obtain direct field observation, information on the ecology and behavior of this elusive species is scarce, with most information based on indirect records (feces, scratches, and footprints) collected in the field (Rheingantz et al., 2017). Studies focusing on communication and general behavior of L. longicaudis corresponded to only 2 and 4%, respectively of all research evaluated with this species in a recent review (de Almeida and Ramos Pereira, 2017). Knowledge on animal behavior enables to develop successful solutions to ex- and in-situ conservation, as well as to propose solutions to wildlife management issues (Campbell-Palmer and Rosell, 2011).
In this study, we document field observations on previously unreported scent marking behavior of L. longicaudis obtained by camera traps. The intra-specific behavior observed in our study can potentially inform ex-situ welfare. Based on our findings we also suggest that habitat use and occupancy data based solely on indirect fieldwork may be biased and should be analyzed carefully for this species.
M\TERIALS AND METHODSStudy area
The study was conducted along 39 km of the Falsino River, in the State of Amapá, Brazil (N 0.77327, W 51.58064; Figure 1). This river segment runs between two sustainable-use protected areas, the Amapá National Forest and the Amapá State Forest (hereafter “FLONA” and “FLOTA”, respectively). This particular stretch of river is 61 km from the nearest town and suffers relatively little anthropogenic influence (Norris and Michalski, 2013; de Oliveira et al., 2015; Norris et al., 2018), with only 3-6 houses in the river segment during our study period.
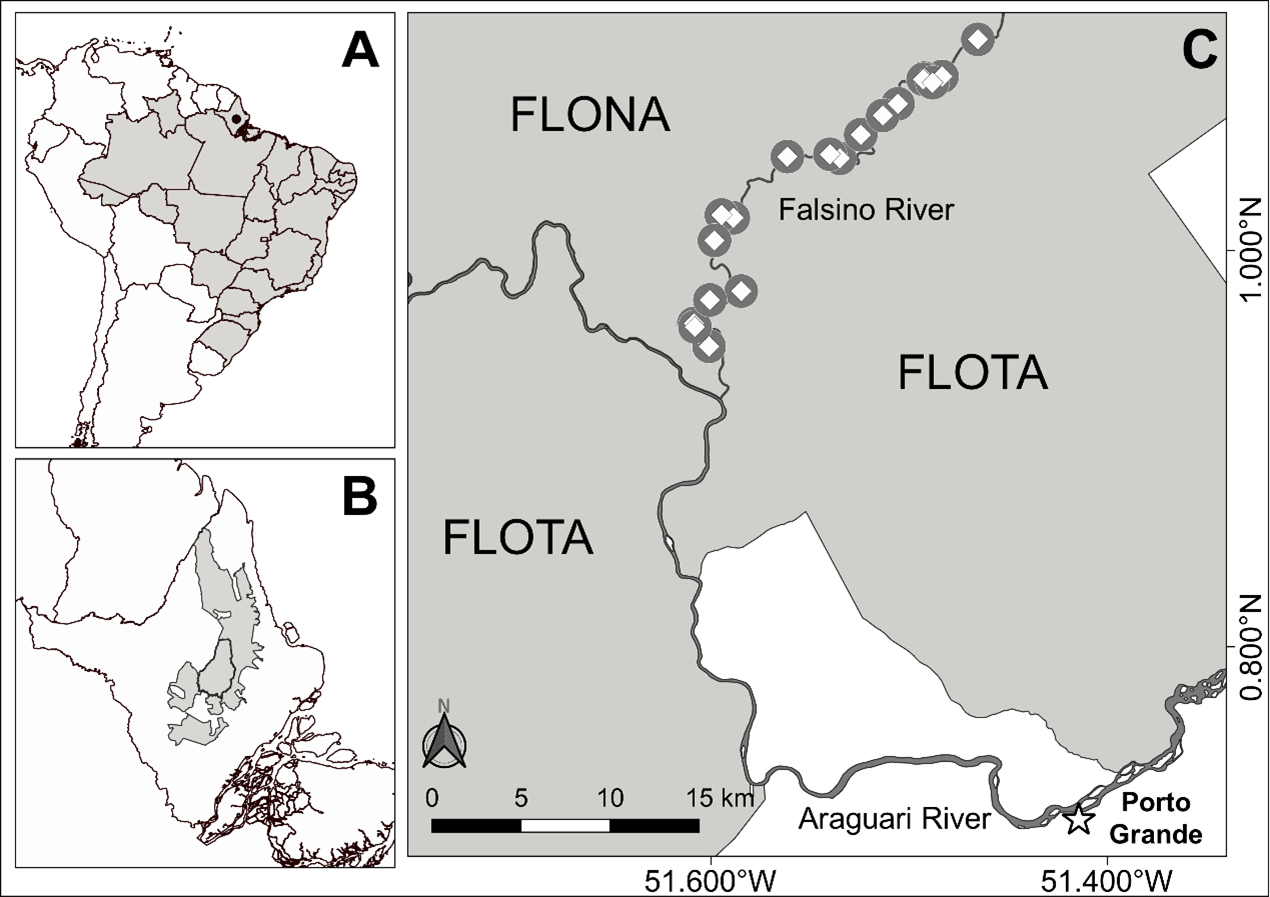
The regional climate is classified by Köppen-Geiger as “Am” (Equatorial monsoon) (Kottek et al., 2006), with the driest months from September to November (total monthly rainfall < 150 mm) and the wettest months from February to April (total monthly rainfall > 300 mm) (Paredes et al., 2017).
Study periods and sampling methods
We obtained data on Neotropical river otter behavior using camera traps equipped with infrared triggers (Bushnell Trophy Cam, 8 MP, Overland Park, KS, USA). Camera traps were installed on islands and river margins during the low river level season (de Oliveira et al., 2015), when islands and sand banks along the river are exposed. Sites were selected based on the following criteria: areas of > 5 m2 of exposed sand and/or fine gravel that were sufficiently raised above the river level not to be waterlogged at a depth of 15 cm (Quintana et al., 2019; Michalski et al., 2020). Sites were not selected to maximize otter encounters or on previous evidence of otter activity. We sampled sites in two consecutive years, with cameras installed in 19 sites from August to December 2018, and in 19 sites from September to December 2019. We maximized the spatial independence between sites by establishing an average (± SD, range) distance along the river of 15.5 km (± 10.4 km, min.-max. =0.06–39.0 km, n=342 comparisons) in 2018, and an average (± SD, range) distance along the river of 15.5 km (± 10.4 km, min.-max. = 0.05–39.0 km, n=342 comparisons) in 2019. Cameras (min.–max. = 1–2 per site) were unbaited, installed at 30-40 cm above the ground and faced the largest open area of the island or margin bank. Cameras functioned continuously (24 hours a day) and were configured in hybrid mode (taking three photos followed by a 40 seconds video film post-activation), with intervals of 15 seconds between videos, and date-time stamp enabled. Here we consider the three photos and video as a single event.
Data analyses
We used the R language with environment for statistical computing (R Development Core Team, 2019) to generate figures and analysis presented in this study. To determine the distances between each site surveyed along the river as well as total river length sampled we used functions available in the R (R Development Core Team, 2019) package “riverdist” (Tyers, 2017). When analyzing data for activity pattern behavior, we only used independent detections, with photos/videos only considered with over 30 minutes interval when the same species was recorded during the same day on the same camera (Michalski et al., 2015; Paredes et al., 2017) or when the individuals could be clearly distinguished using scars, or other visibly unique marks. Information on otter behavior and gender were obtained using frame-to-frame video observations. Gender was confirmed by two researchers (FM and MLR) with over 15 years of experience working with mammals and otters using a double-blinded method.
RESULTS
Following a sampling effort of 2822 trap-days (mean ± SD = 65.6±14.2, range = 38 – 106 per camera trap), we obtained eight independent detections of L. longicaudis (Table 1), with an overall capture rate of 0.3 detections per 100 trap-day.
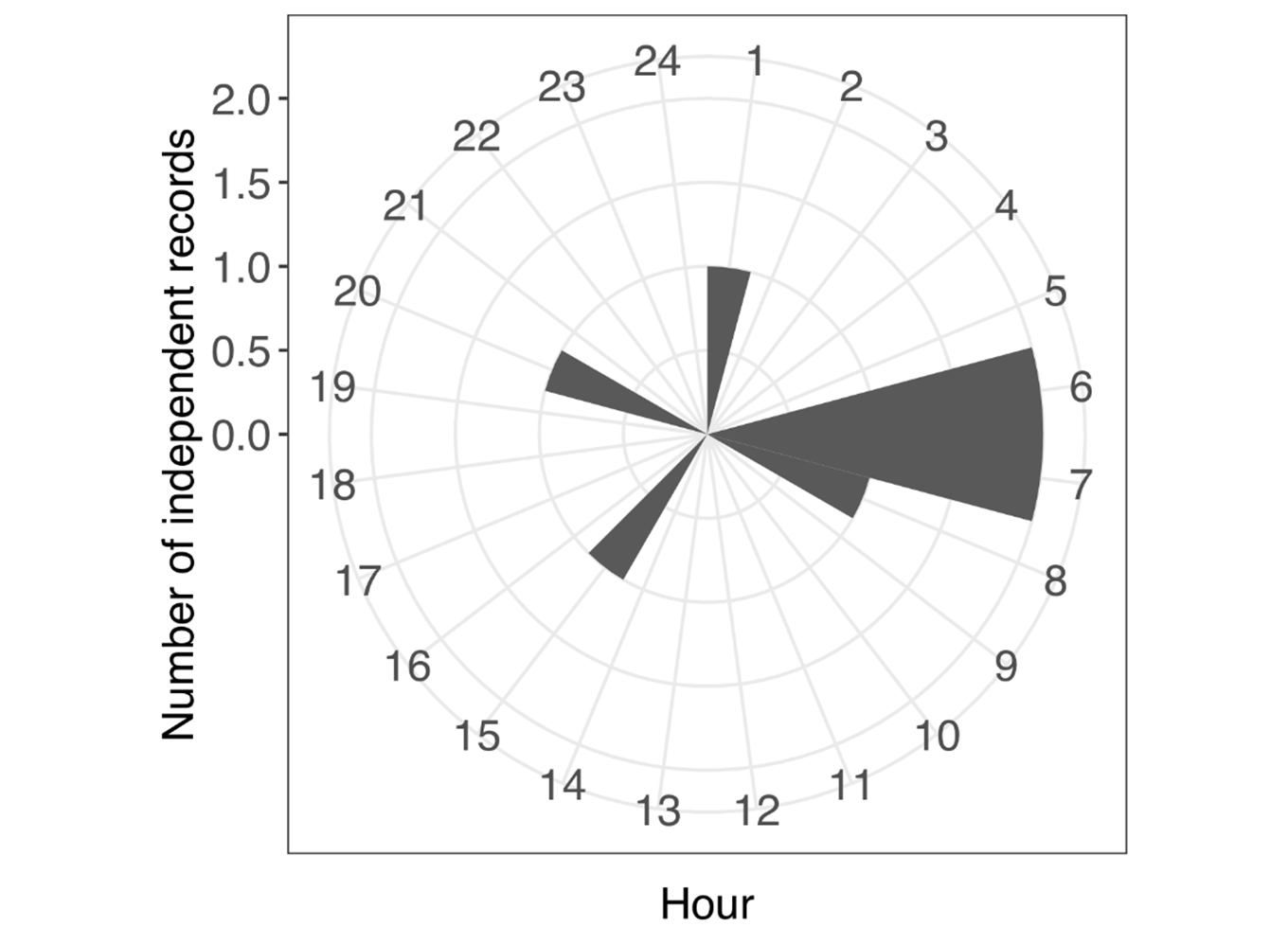
Most of the L. longicaudis detections (75%) were obtained during the day, with only two records detected at night (Figure 2). Half of the records of otters were obtained early in the morning, between 06:00AM and 07:00AM (Figure 2). Solitary adult otters were detected in 62.5% (n=5) of all records and the remaining three events showed two adult otters simultaneously. From the total of eight detections we could only identify gender on four occasions, when the genitalia could be clearly distinguished; being two solitary adult males, and two occasions of male-female couples recorded together.
The majority of otter behavior detected by camera traps was characterized as walking along the sand banks (n=5, 62.5%), but on three occasions (37.5%) we could clearly distinguish behaviors that could be characterized as digging and scent marking, and in one occasion (12.5%) we identified a rubbing behavior.
Digging
Digging is defined as using front paws to remove the substrate forming a shallow crater. In three independent events (19 October 2018 at 06:52AM, 01 December 2018 at 06:22AM, and 05 November 2019 at 07:04AM), adult female and male otters dug the sandy substrate along the river margin. After digging, the otters subsequently returned to the water, with all craters always remaining uncovered/open. During one of these events (01 December 2018), the adult female otter excavated three different craters in less than 20 seconds (Appendix 1). When couples were together, both adult males and females excavated (Appendix 1). In another event (19 October 2018), an adult male otter excavated two different craters in less than 30 seconds on the sandy substrate (Appendix 2).
Scent marking
Scent marking was defined as depositing urine and/or feces on the sandy substrate. Scent marking with urine inside the craters after digging was clearly identified during two events. This behavior was detected once with a solitary male and once with a couple. In one event (05 November 2019 at 07:04AM), the scent marking was done with feces, which were left clearly on the surface of the substrate by a male (Appendix 3). On another event (01 December 2018 at 06:22AM), we detected an interaction behavior between two adults; when a female clearly deposited her urine inside at least two craters that she had freshly dug, and the male immediately (< 8 second interval) deposited his urine on top of the female’s urine in the same two craters (Appendix 1).
Rubbing
Rubbing was defined when an animal rubs its belly and genitalia/anal gland on the sandy substrate. Rubbing was identified in one event (19 October 2018 at 06:52AM) when an adult male exhibited belly and genitalia rubbing on the sandy substrate on the river margin twice (Appendix 2).
DISCUSSION
As far as we are aware this is the first study to document the digging followed by scent marking behavior in wild Neotropical Otters. Due to their secretive nature and the difficulty of directly observing individuals in the field (de Almeida and Ramos Pereira, 2017; Rheingantz et al., 2017) many behavioral aspects of this semi-aquatic species remain unknown. We first turn to discuss the low number of detections, and then explore information on activity patterns and number of simultaneously detected individuals. Finally, we discuss how the digging, scent marking, and rubbing behavior observed in our study helps to increase the knowledge of the species and inform both in-situ and ex-situ management and conservation initiatives.
We found a low detection rate (0.3 detections per 100 trap-days) of Neotropical Otters in our cameras located on islands and sand banks along 39 km of river. This low detection rate is perhaps surprising, considering the intensive sampling effort (2822 trap-days from 19 sites in 2018 and 19 sites in 2019), within a relatively short river segment (39 km) in our study. Thus, our results corroborate previous studies that found this species to be difficult to obtain direct detections in the field (Rheingantz and Trinca, 2015; de Almeida and Ramos Pereira, 2017; Rheingantz et al., 2017). This could be a reflection of the fact that our cameras were installed in dry exposed areas along the river, which are not necessarily frequently used by Neotropical Otters, a species that depends predominantly on water bodies for feeding and foraging activities (Kruuk, 2006). Moreover, camera trap studies only had higher number of detections of Neotropical Otters when cameras were placed facing otter dens (Rheingantz et al., 2016). As our study area has low levels of anthropogenic disturbance (Norris and Michalski, 2013; de Oliveira et al., 2015; Quintana et al., 2019), and has the presence of the entire community of vertebrates (Michalski et al., 2015; Paredes et al., 2017), we would expect that Neotropical Otters could be more easily detected in our region when compared with more fragmented and disturbed areas in the Brazilian Amazon (Michalski and Peres, 2005), Pantanal or Atlantic Forest (Rheingantz et al., 2016).
Although our records do not fully represent the activity pattern of L. longicaudis in our study area, our results with records obtained during both day and night, corroborate the plasticity of activity already described in the literature (Nakano-Oliveira et al., 2004; Rheingantz et al., 2016). But we found otters to be more active during the day, with a peak of records during the first hours of the morning, which was already recorded in the Brazilian Atlantic forest and in the Pantanal (Rheingantz et al., 2016). Similarly, most of the records obtained in our study were composed of single adults walking and inspecting islands and sand banks along the river. The solitary behavior of this species, with only occasional records of adults in pairs or in small groups of females and their cubs was already reported in the literature (Rodrigues et al., 2013; Rheingantz and Trinca, 2015; Rheingantz et al., 2017).
Although otters are known to use scent marking to communicate (Kruuk, 1992, Ben-David et al., 2005), and Neotropical Otters (Lontra longicaudis) are already known to use scent marking as communication between individuals (Rheingantz and Trinca, 2015; Rheingantz et al., 2017), the behavior of digging and depositing urine in exposed craters has not been described before. Neotropical Otters deposit feces and mucus in conspicuous locations such as rocks, fallen tree trunks, and sand banks along rivers (Dunstone and Strachan, 1988; Rheingantz et al., 2016; 2017), but until now, the behavior of individuals depositing urine on top of each other’s, which is probably linked with sexual behavior (Larivière, 1999) has not been recorded previously. Similarly, rubbing behavior, with males rubbing belly and genitalia on sandy substrates along river margins has not been described before. Rubbing behavior in mammals is less frequently documented in the literature when compared to marking with urine or feces but some studies already documented rubbing in mammals (Bel et al., 1999; Allen et al., 2014; 2017).
CONCLUSIONS
Our study contributes with new information on the behavior of this species of river otter, which has lack of information on behavior ecology (de Almeida and Ramos Pereira, 2017). We also bring information that could potentially help animal welfare in zoos, as sandy substrate can be important for otter communication. Animal welfare has been a current focus in research and information coming from elusive and free-ranging animals such as Neotropical Otters seems to be crucial and difficult to obtain. Thus, we believe our study can benefit conservation ex-situ and contribute with better knowledge on ecological behavior of Neotropical Otters.
Finally, our data on otter use of islands and sand banks along Amazonian rivers suggest that studies based solely on indirect signs (e.g., feces, footprints) to predict habitat use and anthropogenic effects on Neotropical Otters may be biased and must be evaluated with caution. Ideally, studies must use a combination of different methods using direct and indirect detections to model habitat use by river otters (Gomez et al., 2014), which may reduce biases related with the lack of detection of otters in easily washed records such as footprints.
Acknowledgements: Funding was provided by the United States National Academy of Sciences and the United States Agency for International Development through the Partnership for Enhanced in Research (http://sites.nationalacademis.org/pga/peer/index.htm) award number AID-OAA-A11-00012 to DN and FM. Additional funding was provided by Conservation, Food & Health Foundation awarded to FM. FM receives a productivity scholarship from the National Council for Scientific and Technological Development (CNPq – process 302806/2018-0) and is funded by CNPq (process 403679/2016-8). CBM receives a MSc scholarship from the CNPq (process 132558/2019-0). DN is funded by CNPq (process 433638/2018-4). The Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO) and the Universidade Federal do Amapá provided logistical support. We thank the IBAMA for authorization to conduct research in FLONA (IBAMA/SISBIO permits 49632-1, 63668-1, and 63668-2). We are grateful to Alvino Pantoja Leal and Gilberto dos Santos Souza for their invaluable assistance during fieldwork. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript. The authors declare no conflict of interest.
REFERENCES
Allen, M.L., Gunther, M.S., Wilmers, C.C. (2017). The scent of your enemy is my friend? The acquisition of large carnivore scent by a smaller carnivore. Journal of Ethology 35(1): 13-19.
Allen, M.L., Wittmer, H.U., Houghtaling, P., Smith, J., Elbroch, L.M., Wilmers, C.C. (2015). The Role of Scent Marking in Mate Selection by Female Pumas (Puma concolor). Plos One 10(10): e0139087.
Allen, M.L., Wittmer, H.U., Wilmers, C.C. (2014). Puma communication behaviours: understanding functional use and variation among sex and age classes. Behaviour 151: 819-840.
Allen, M.L., Yovovich, V., Wilmers, C.C. (2016). Evaluating the responses of a territorial solitary carnivore to potential mates and competitors. Scientific Reports 6(1): 27257.
Bel, M.C., Coulon, J., Sreng, L., Allaine, D., Bagneres, A.G., Clement, J.L. (1999). Social signals involved in scent-marking behavior by cheek-rubbing in Alpine marmots (Marmota marmota). Journal of Chemical Ecology 25: 2267-2283.
Ben-David, M., Blundell, G.M., Kern, J.W., Maier, J.A.K., Brown, E.D., Jewett, S.C. (2005). Communication in river otters: Creation of variable resource sheds for terrestrial communities. Ecology 86(5): 1331-1345.
Campbell-Palmer, R., Rosell, F. (2011). The importance of chemical communication studies to mammalian conservation biology: A review. Biological Conservation 144(7): 1919-1930.
Carter, S.K., Rosas, F.C.W. (1997). Biology and conservation of the Giant Otter Pteronura brasiliensis. Mammal Review 27(1): 1-26.
de Almeida, L.R., Ramos Pereira, M.J. (2017). Ecology and biogeography of the Neotropical otter Lontra longicaudis: existing knowledge and open questions. Mammal Research 62(4): 313-321.
de Oliveira, I.A.P., Norris, D., Michalski, F. (2015). Anthropogenic and seasonal determinants of giant otter sightings along waterways in the northern Brazilian Amazon. Mammalian Biology 80(1): 39-46.
Dunstone, N., Strachan, R. (1988). Status and distribution of otters in the Amboro National Park, Bolivia. IUCN Otter Specialist Group Bulletin 3: 24-31.
Gese, E.M., Ruff, R.L. (1997). Scent-marking by coyotes, Canis latrans: the influence of social and ecological factors. Animal Behaviour 54(5): 1155-1166.
Gomez, J.J., Tunez, J.I., Fracassi, N., Cassini, M.H. (2014). Habitat suitability and anthropogenic correlates of Neotropical river otter (Lontra longicaudis) distribution. Journal of Mammalogy 95(4): 824-833.
Johnson, R.P. (1973). Scent marking in mammals. Animal Behaviour 21(3): 521-535.
Kean, E.F., Müller, C.T., Chadwick, E.A. (2011). Otter Scent Signals Age, Sex, and Reproductive Status. Chemical Senses 36(6): 555-564.
Kottek, M., Grieser, J., Beck, C., Rudolf, B., Rubel, F. (2006). World map of the Koppen-Geiger climate classification updated. Meteorologische Zeitschrift 15(3): 259-263.
Kruuk, H. (1992). Scent marking by otters (Lutra lutra): signaling the use of resources. Behavioral Ecology 3(2): 133-140.
Kruuk, H. (2006). Otters: Ecology, Behaviour and Conservation (1st ed.). Oxford, UK: Oxford University Press.
Kruuk, H. (2014)b. Sprainting Into The Wind. IUCN Otter Specialist Group Bulletin 31(1): 12-14.
Larivière, S. (1999). Lontra longicaudis. Mammalian Species 609(5): 1-5.
Leuchtenberger, C., Mourão, G. (2009). Scent-Marking of Giant Otter in the Southern Pantanal, Brazil. Ethology 115(3): 210-216.
MacDonald, D.W. (1980). Patterns of scent marking with urine and faeces amongst carnivore communities. Paper presented at the Symposia of the Zoological Society of London.
Michalski, F., Norris, D., Quintana, I., Valerio, A., Gibbs, J.P. (2020). Substrate influences human removal of freshwater turtle nests in the eastern Brazilian Amazon. Scientific Reports 10(1): 8082.
Michalski, F., Peres, C.A. (2005). Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biological Conservation 124(3): 383-396.
Michalski, L.J., Norris, D., de Oliveira, T.G., Michalski, F. (2015). Ecological Relationships of Meso-Scale Distribution in 25 Neotropical Vertebrate Species. Plos One 10(5): e0126114.
Nakano-Oliveira, E., Fusco, R., Santos, E.A.V., Monteiro-Filho, E.L.A. (2004). New information about the behavior of Lontra longicaudis (Carnivora: Mustelidae) by radio-telemetry. IUCN Otter Specialist Group Bulletin 21(1): 21-23.
Norris, D., Michalski, F. (2013). Socio-economic and spatial determinants of anthropogenic predation on Yellow-spotted River Tuttle, Podocnemis unifilis (Testudines: Pelomedusidae), nests in the Brazilian Amazon: Implications for sustainable conservation and management. Zoologia 30(5): 482-490.
Norris, D., Michalski, F., Gibbs, J.P. (2018). Community involvement works where enforcement fails: conservation success through community-based management of Amazon river turtle nests. Peerj 6: e4856.
Paredes, O.S.L., Norris, D., de Oliveira, T.G., Michalski, F. (2017). Water availability not fruitfall modulates the dry season distribution of frugivorous terrestrial vertebrates in a lowland Amazon forest. Plos One 12(3): e0174049
Piñeiro, A., Barja, I. (2015). Evaluating the function of wildcat faecal marks in relation to the defence of favourable hunting areas. Ethology Ecology & Evolution 27(2): 161-172.
Quintana, I., Norris, D., Valerio, A., Becker, F.G., Gibbs, J.P., Michalski, F. (2019). Nest removal by humans creates an evolutionary trap for Amazonian freshwater turtles. Journal of Zoology 309(2): 94-105.
R Development Core Team. (2019). R Development Core Team. R 3.6.0: A language and environment for statistical computing. Vienna, Austria.
Ralls, K. (1971). Mammalian scent marking. Science 171(3970): 443-449.
Rheingantz, M.L., Leuchtenberger, C., Zucco, C.A., Fernandez, F.A.S. (2016). Differences in activity patterns of the Neotropical otter Lontra longicaudis between rivers of two Brazilian ecoregions. Journal of Tropical Ecology 32: 170-174.
Rheingantz, M.L., Santiago-Plata, V.M., Trinca, C.S. (2017). The Neotropical otter Lontra longicaudis: a comprehensive update on the current knowledge and conservation status of this semiaquatic carnivore. Mammal Review 47(4): 291-305.
Rheingantz, M.L., Trinca, C.S. (2015). Lontra longicaudis. The IUCN Red List of Threatened Species 2015: e.T12304A21937379. Retrieved from https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12304A21937379.en.
Roberts, N.J., Clark, R.M., Williams, D. (2016). Otter (Lontra longicaudis) spraint and mucus depositions: early ecological insights into the differences in marking site selection and implications for monitoring prey availability. IUCN Otter Specialist Group Bulletin 33(1): 8-17.
Rodrigues, L.A., Leuchtenberger, C., Kasper, C.B., Carvalho-Junior, O., Silva, V.C.F. (2013). Avaliação do risco de extinção da Lontra Neotropical. Biodiversidade Brasileira 3(1): 216-227.
Roper, T.J., Conradt, L., Butler, J., Christian, S.E., Ostler, J., Schmid, T.K. (1993). Territorial Marking with Faeces in Badgers (Meles meles): A Comparison of Boundary and Hinterland Latrine Use. Behaviour 127(3/4): 289-307.
Rostain, R.R., Ben-David, M., Groves, P., Randall, J.A. (2004). Why do river otters scent-mark? An experimental test of several hypotheses. Animal Behaviour 68: 703-711.
Smith, J.L.D., McDougal, C., Miquelle, D. (1989). Scent marking in free-ranging tigers, Panthera tigris. Animal Behaviour 37: 1-10.
Thiessen, D., Rice, M. (1976). Mammalian scent gland marking and social behavior. Psychological Bulletin 83(4): 505-539.
Tyers, M. (2017). Tyers, M. riverdist: river network distance computation and applications. R package version 0.15.0. Retrieved from https://cran.r-project.org/web/packages/riverdist/index.html
APPENDICES
Appendix 1 - Digging and scent marking behavior of Lontra longicaudis. Two adults (one female and one male) scent marking the margin of a sand bank along the river. The female excavates and urine in the burrow and after the male urines on top of the female’s urine.
Appendix 2 - Digging, scent marking, and rubbing behavior of Lontra longicaudis. One adult male scent marking and rubbing its belly and genitalia on the margin of a sand bank along the river.
Appendix 3 - Digging and scent marking behavior of Lontra longicaudis. Two adults (one female and one male) scent marking the margin of a sand bank along the river. The male excavates, smells, and deposit and left feces in a clear place on the substrate.
Résumé: Nouveau Comportement de Marquage Olfactif chez la Loutre à Longue Queue (Lontra longicaudis) dans L’Est de l’Amazonie Brésilienne
Le comportement de marquage olfactif chez les mammifères est lié à la fois à la communication inter et intra-spécifique. Plusieurs espèces de loutres sont connues pour communiquer via un marquage olfactif, mais un marquage olfactif d’un couple n'a pas été observé chez la loutre à longue queue (Lontra longicaudis). Nous obtenons des observations de terrain sur le comportement de marquage olfactif des loutres à longue queue, durant deux ans, à l'aide de pièges photographiques, le long des cours d'eau dans l'est de l'Amazonie brésilienne. Nos résultats révèlent l'utilisation de substrats sableux sur les îles et les berges des rivières pour la communication intra-spécifique entre loutres. La plupart des enregistrements (62,5%) proviennent d'adultes solitaires. Nous observons plusieurs enregistrements distincts de loutres adultes creusant pour flairer l'urine et le comportement de couple de mâles urinant sur l'urine fraîche de la femelle dans des dépressions peu profondes récemment creusées. Nous démontrons également la plasticité comportementale de cette espèce mise en évidence par des pièges photographiques enregistrant l'activité terrestre de jour comme de nuit. Nos résultats contribuent à améliorer la connaissance du comportement de cette espèce de loutre dans la nature et peuvent potentiellement être appliqués pour améliorer le bien-être ex situ des loutres captives.
Revenez au dessus
Resumen: Nuevo Comportamiento de Marcación Olorosa de la Nutria Neotropical (Lontra longicaudis) en la Amazonia Oriental Brasilera
El comportamiento de marcación olorosa en los mamíferos se relaciona tanto con la comunicación inter-específica como la intra-específica. Varias especies de nutrias se sabe que se comunican mediante marcación olorosa, pero la marcación olorosa por parejas no ha sido documentada en la Nutria Neotropical (Lontra longicaudis). Obtuvimos observaciones de terreno del comportamiento de marcación olorosa en Nutrias Neotropicales a lo largo de dos años utilizando cámaras-trampa a lo largo de cursos de agua en la Amazonía oriental brasilera. Nuestros resultados revelan el uso de sustratos arenosos en islas y márgenes de ríos para la comunicación intra-específica entre nutrias. La mayoría de los registros (62.5%) fueron de adultos solitarios. Documentamos múltiples registros independientes de nutrias adultas excavando para marcar con olor, con orina, y comportamiento de pareja de machos orinando encima de orina fresca de hembras en cráteres poco profundos recién excavados. También demostramos la plasticidad comportamental de esta especie, evidenciada por los registros con cámara-trampa, de actividad terrestre tanto diurna como nocturna. Nuestros resultados contribuyen a mejorar el conocimiento del comportamiento de esta especie de nutria en la naturaleza, y pueden ser potencialmente aplicados al mejoramiento del bienestar ex-situ de nutrias en cautiverio.
Vuelva a la tapa



