IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 4 (August 2021)
Citation: Damasceno, J. de S., Delponte, J.C. and Shiralwa, M.C.S. (2021). Dietary Adaptability of the Giant Otter, Pteronura brasiliensis (Mammalia: Mustelidae), in two Floodplain Systems in the Pantanal Wetland, Mato Grosso State, Brazil. IUCN Otter Spec. Group Bull. 38 (4): 202 - 216
Dietary Adaptability of the Giant Otter, Pteronura brasiliensis (Mammalia: Mustelidae), in two Floodplain Systems in the Pantanal Wetland, Mato Grosso State, Brazil
Júnio de Souza Damasceno1,2*, Júlio César Dalponte3,4 and Marília Couto Silva Shiraiwa2
1Pontifícia Universidade Católica de Minas Gerais (PUC Minas), Programa de Pós-Graduação em Biologia de Vertebrados. Av. Dom José Gaspar, 500, Coração Eucarístico, CEP: 30535-901, Belo Horizonte, MG, Brazil.
2Universidade Federal de Mato Grosso (UFMT), Programa de Pós Graduação em Ecologia e Conservação da Biodiversidade. Av. Fernando Correa da Costa, 2367, Coxipó, CEP: 78060-900, Cuiabá, MT, Brazil
3Universidade Federal de Mato Grosso (UFMT), Campus de Sinop. Av. Alexandre Ferronato, 1.200, Bairro: Setor Industrial, CEP: 78550-000 – Sinop, MT, Brazil
4Instituto Pró-Carnívoros, Av. Horácio Netto, 1030 - Chácaras Interlagos, Atibaia - SP, 12945-010
* Corresponding Author Email: jdamascenobh@gmail.com
(Received 6th July 2020, accepted 23rd February 2021)
Abstract: The giant otter, Pteronura brasiliensis, is an almost exclusively piscivorous mammal of the family Mustelidae, and an endangered species. The present study compared the diet of the giant otter in two floodplain systems of the Pantanal wetland, a permanent lake and a river branch, in the Poconé and Barão de Melgaço wetlands in the Brazilian state of Mato Grosso. Samples were collected during the dry seasons, with 43 spraints being collected from the lake and 31 from the river branch. The fish species present in the samples were identified based on the comparison of bone fragments found in the spraints with specimens of fish collected from the two floodplain systems. The diets were composed primarily of fish of the families Erythrinidae (94.6%), Cichlidae (91.9%), Pimelodidae (70.3%), Callichthyidae (63.5%), and Doradidae (60.8%). Catfish (Siluriformes) were more abundant in the samples from the lake, while characins (Characiformes) were well represented in the river branch. While the siluriform family Callichthyidae was a prominent component of the otter diet in both study areas, it has been rarely recorded in previous studies of P. brasiliensis in either the Amazon or Pantanal regions. Most of the fish recorded in the diet of the otter are not targeted by commercial fisheries in Mato Grosso. The varying characteristics of different aquatic systems are important determinants of the composition of the local fish fauna and are thus relevant to the feeding ecology of the giant otter in the Pantanal wetland.
Keywords: Neotropical biodiversity, Pantanal, Pteronura brasiliensis, latrine, feeding ecology, ichthyofauna
INTRODUCTION
The giant otter, Pteronura brasiliensis (Gmelin, 1788), is a predominantly piscivorous mammal, which feeds opportunistically on the most abundant fish found near the margins of rivers and lakes (Duplaix, 1980; Schweizer, 1992; Carter and Rosas, 1997; Paglia et al., 2012; Duplaix et al., 2015). These otters rarely consume other types of prey (Cabral et al., 2010; Silva et al., 2013). Most studies of the feeding ecology of the giant otter are based on the identification and quantification of the hard parts of prey species found in spraints (Laidler, 1984; Rosas et al., 1999; Rosas-Ribeiro et al., 2012), although some direct observations have been possible (Duplaix, 1980). However, direct observation is logistically more complex and costly than fecal analysis (Carter et al., 1999).
Excessive hunting for the fur trade has had a fundamental impact on giant otter numbers (Carter and Rosas, 1997), and in 2000, the conservation status of P. brasiliensis was upgraded from “Vulnerable” to “Endangered” by the International Union for Conservation of Nature (Duplaix et al., 2008), and the species has remained in this category ever since (Groenendijk et al., 2015). The ongoing accumulation of human pressures and habitat loss in South America is the principal threat to this species (Groenendijk et al., 2015). Tourism and conflicts with fishers have also had an important impact on the species in the Pantanal wetland of Brazil (Rosas et al., 2008). In Brazil, P. brasiliensis is classified as “Vulnerable” in the Official List of Species of Brazil Threatened with Extinction (MMA, 2018).
This degree of threat reinforces the need for ecological studies that consolidate the understanding of the ecology of P. brasiliensis and provide an important database for the conservation of the giant otter. While the giant otter populations persist within the historical range of the species, the illegal fur trade, conflicts with fisheries, mining, the construction of dams, tourism, deforestation, and climate change have all intensified the pressures on the populations remaining in the wild (Duplaix et al. 2015).
Giant otter populations in the Pantanal wetland have been the focus of a number of important studies, including genetics and conservation (Garcia et al., 2007; Pickles et al., 2012), habitat characteristics (Camilo-Alves and Desbiez 2005), contamination by mercury (Fonseca et al., 2005), and ethology (Leuchtenberger and Mourão, 2008; Leuchtenberger et al., 2013). Schweizer (1992), Rosas et al. (1999) and Leuchtenberger et al., (2020) have provided some data on the diet of the giant otter in the southern Pantanal, but systematic studies of the species’ diet and other aspects of its ecology in the northern Pantanal are scarce. In addition, the relative contribution of lakes and creeks to the diet of P. brasiliensis has been poorly documented, not only in the Pantanal, but also in other regions.
The present study compares the diets of P. brasiliensis in two different types of aquatic system that are common in the northern Pantanal wetland in the state of Mato Grosso, Brazil. One type of system is the permanent lakes, known locally as “baías”, while the other is branches of rivers, known as “corixos”. The study analyzes differences between these systems in the taxonomic composition of the P. brasiliensis diet in these two systems during the dry season. The data were also compared with those collected in previous studies of the diet of giant otters in the Amazon regions (Cabral et al., 2010); Rosas-Ribeiro et al., 2012; Silva et al., 2013) and Pantanal (Rosas et al., 1999; Leuchtenberger et al., 2020).
MATERIAL AND METHODS
Study area
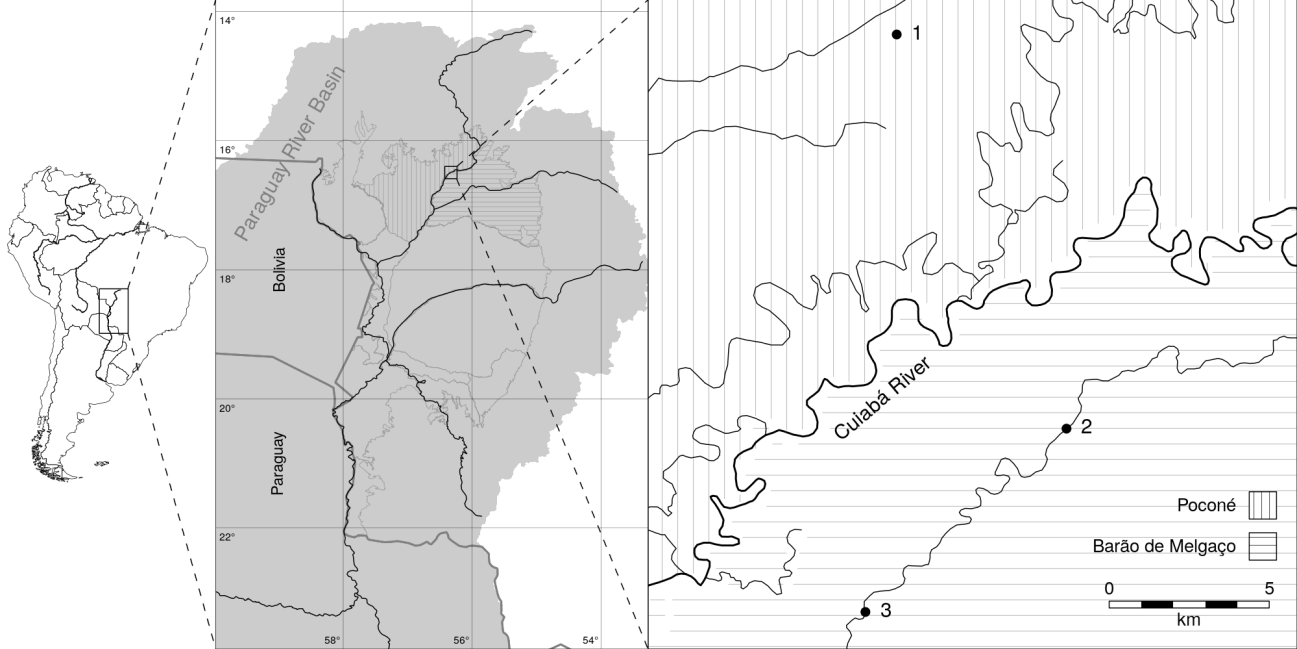
The present study was conducted in the northern Pantanal, in the southwestern extreme of the Brazilian state of Mato Grosso, which covers a total area of 147,574 km2 of wetland habitats, formed by the Paraguay River and its tributaries (15º30’–22º30’ S, 54º45’–58º30’ W). The Pantanal encompasses an enormous diversity of riparian habitats and forest fragments that are flooded either permanently or over the course of the annual flood cycle (Alho et al., 2008). The drainage capacity of the floodplain is greatly reduced, with water accumulating in depressions, forming lakes (baías) or being flooded permanently. During the flood period, the water flows slowly through channels known locally as “corixos” or along shallow runoffs, known as “vazantes” (Alho and Sabino 2012).
The annual flood pulse of the Pantanal floodplain is the principal driver of changes in the composition of the local fish communities, with a high level of species turnover occurring between the flood and dry seasons. This marked temporal gradient in the hydrological cycle provokes a large-scale response in the local fish communities to the annual flood pulse (Pains da Silva et al., 2010).
The study was conducted in two distinct types of habitat, a permanent lake and a river branch. While small drainage channels or river branches (corixos) persist on the floodplain during the dry season, connecting rivers and lakes, in general, the permanent lakes (baías) only receive an inflow of water during the flood season and remain isolated from other bodies of water in the dry season (Nunes da Cunha and Junk, 2004).
Baía das Pedras (16º24’36.7” S, 56º21’08.6” W) is one of the lakes found in a locality known as Pirizal, in the Poconé wetland, in the municipality of Nossa Senhora do Livramento. This lake is connected to the Piraim River, a right-margin tributary of the Cuiabá River, during the flood season, when it receives an increased load of organic matter, which has a considerable impact on the structure of the ecosystem (Nogueira et al., 2002). Baía das Pedras Lake has a surface area of 2.08 ha and a perimeter of 10,750 m, with a maximum depth of 6.2 m, mean depth of 1.04 m, maximum effective width of 100 m, and maximum effective length of 274 m (Nogueira et al., 2002).
The Corixo Espírito Santo is part of the SESC Private Natural Heritage Reserve, which, in turn, is located within the SESC–Pantanal Ecological Station (16–17º S, 56–57º W), with a total area of 106,588 ha, located in the northern Pantanal wetland (Antas et al., 2011). The length of this corixo is regulated by the flood pulse. In the dry season of 2002, the corixo had a length of 13 kilometers (16º31’ S, 56º17’ W to 16º34’ S, 56º21’ W), with two small branches of one kilometer in length (16º33’53” S, 56º20’ W and 16º33’ S, 56º20’ W). This corixo is 100–200 m wide during the dry season, with gently sloping margins covered predominantly by dense arboreal and shrubby vegetation. In some stretches, there are sandy beaches and flat sandbanks covered with grass as far as the waterline (Figure 1).
A giant otter group has been observed in Baía das Pedras Lake (Pinho, JB., personal communication). In addition, recent sightings of active giant otter dens and individuals have been found in Corixo Espírito Santo (Neves, C.C. personnal communication), and thus they are relevent to conservation of P. brasiliensis in the Pantanal This biome has been affected by progressive loss of natural habitat through deforestation and fires. More than 20,000 fire alerts were registered in 2020; three times the historical average (1998-2020) (INPE, 2020). From 1988 to 2019, Mato Grosso state had already deforested 146,140 km2 of natural habitat (Shimabukuro et al., 2020), a lost natural cover area equivalent to Pantanal along the last 30 years.
The vegetation cover of the study area is predominantly savannah, with diverse phytophysiognomies in both floodable and non-flooded areas. The fauna is diverse and abundant and includes a number of endangered species (Alho et al., 2019). The flood-pulse reflects the cycle of rainy and dry seasons typical of the Pantanal wetland (Ivory et al., 2019). Therefore, extreme climatic events (Thielen et al., 2020) and conversion of natural ecosystems (Colman et al. 2019) are altering patterns of the hydrological and sediment dynamics, disrupting local ecology and the socioeconomic relationships of the basin (Schulz et al., 2019). Two larger rivers, the Cuiabá River and the São Lourenço River, are found in the marginal areas of the Natural Heritage Private Reserve (Antas et al., 2016).
Spraint collection and analysis
The diet of the giant otter was compared between sites based on the analysis of spraints collected from otter latrines during the dry seasons (July to December) of 2002 and 2003. Each sample was placed in a numbered plastic bag, and then washed and dried for the identification of the components (animal fragments), following Rosas et al. (1999). Only fresh spraints, recognized by their characteristic color and odor, were collected, with a total of 74 samples being accumulated over the study period.
In Baía das Pedras Lake spraints were collected from a group of eight giant otters (four adults and four cubs) in four communal latrines. In Corixo Espírito Santo spraints were collected from groups, being a group of eight individuals (five adults and three cubs) and other group consisted five adult individuals from 14 giant otter communal latrines.
The fragments of fish found in the spraints were sorted and analyzed, with the species being identified using taxonomic keys (Britski et al., 1999), comparisons with fish specimens collected from the study area, and consultation with specialists.
The fragments analyzed for identification were divided into five types of structure: scales, opercula, mandibles, vertebrae, and miscellaneous bone structures (vertebrae, skull and fin bones, and carapaces). The mollusks (Pomacea) were identified from their carapaces, and the crustaceans from their appendages (Magalhães, 2000). Only fragments longer than 1 cm were considered, in order to minimize the probability of overestimating the number of items present in each sample. The fragments of fish bones identified in this analysis were deposited in a reference collection at the vertebrate collection of the Bioscience Institute of the Federal University of Mato Grosso in Cuiabá (Brazil).
The percentage of occurrence of each prey group was determined for all the spraints. A contingency table (G test) was used to test the hypothesis that the composition of the diet (prey categories) is independent of the environment (lake vs. river branch). The G test, with Williams’ correction (Zar, 1996), was chosen for this analysis because some cells of the contingency table had expected frequencies of 5 or less. This procedure was complemented with an analysis of the residuals, to determine the probabilistic contribution of each cell (i.e., the prey categories).
The frequencies of items of the three orders of fish (Characiformes, Siluriformes and Perciformes) found in the spraints collected in the present study were also compared with the corresponding frequencies recorded in the Amazon (Rosas et al., 1999; Cabral et al., 2010; Rosas-Ribeiro et al., 2012; Silva et al., 2013) and Pantanal regions (Rosas et al., 1999; Leuchtenberger et al., 2020). This analysis was also based on the G test, as described above following a calculate p-value partitioning chi-square test. The analyses were run in BioEstat 5.0 (Ayres et al., 2007).
RESULTS
Seventy-four giant otter spraints (43 from Baía das Pedras Lake and 31 from the Corixo Espírito Santo) were analyzed here (Table 1). In both areas, the diet was composed of fish, crustaceans, and mollusks. Fish were present in 100% of the samples at both sites. Crustaceans were found in four samples (9.3%) from Baía das Pedras, and mollusks were found in nine samples (20.9%). At Corixo Espírito Santo, crustaceans and mollusks were each found in only a single sample (3.2%).
The five most frequent families of fish recorded at both sites were the Erythrinidae (found in 94.6% of the spraints), Cichlidae (91.9%), Pimelodidae (70.3%), Callicthyidae (63.5%), and Doradidae (60.8%). The contributions of the different taxa to the otter diet varied between sites, however. At Baía das Pedras, three fish orders (Characiformes, Siluriformes and Perciformes) were recorded in the spraints. In the case of the Characiformes, the family Erythrinidae, represented primarily by Hoplias malabaricus (Bloch, 1794), was present in 95.3% of the samples, followed by the family Serrasalmidae (67.4%). The order Siluriformes was well represented by three families, the Callicthyidae (97.7% of the samples), Doradidae (95.3%), and Pimelodidae (93.0%), while the order Perciformes was represented only by the family Cichlidae, which was present in 100% of the samples and was the most frequent prey category in the Baía das Pedras samples.
At Corixo Espírito Santo, characiforms were recorded in all 31 samples and this order was represented primarily by the family Erythrinidae (93.5% of the samples), followed by the Serrasalmidae (48.4%). In contrast with Baía das Pedras, siluriforms were present in only 54.8% of the samples, with pimelodids being found in 38.7%. of the spraints, while the Doradidae and Callicthyidae were each present in 12.9% of the samples. The only perciform family recorded at this site was the Cichlidae, present in 25 samples (80.6%).
Significant differences were found between the lake and corixo in the numbers of the principal items found in the spraints (G test, 14 x 2 contingency table; G = 52.919; P< 0.0001). The results of the residual analysis indicate that the spraints from the lake had a higher frequency of siluriforms and relatively lower frequency of perciforms and erythrinids (Characiformes) than the corixo (Table 2). The frequencies of the other characiforms were similar at the two sites (Table 3 ).
| Table 3 : Frequency of occurrence (number of times a prey item was recorded in the spraints) of the three principal orders of fish consumed by the giant otter (Pteronura brasiliensis) at eight Brazilian study localities: Baía das Pedras and Corixo Espírito Santo (present study); Xixuaú Creek and Aquidauana River (Rosas et al. 1999), Balbina reservoir (Cabral et al., 2010); Juruá River (Rosas-Ribeiro, et al., 2012), the PARNA Jaú (Silva et al., 2013) and Corumbá Pantanal (Leuchtenberger et al., 2020). The percentage of spraints containing each item is shown in parentheses. | ||||
| Site | Taxonomic order of fish | Study | ||
| Characiformes | Perciformes | Siluriformes | ||
| Xixuaú Cove (RR) | 32 (100) | 36 (97.3) | 2 (20.0) | Rosas et al., (1999) |
| Aquidauana River (MS) | 6 (100) | 4 (33.3) | 2 (66.6) | Rosas et al., (1999) |
| Balbina Dam (AM) | 197 (77.5) | 236 (92.9) | 16 (6.3) | Cabral et al., (2010) |
| Juruá River (AM) | 92 (86.6) | 25 (23.6) | 31 (29.1) | Rosas-Ribeiro et al., (2012) |
| Jaú National Park (AM) | 69 (84.1) | 75 (91.5) | 18 (22.0) | Silva et al., (2013) |
| Corumbá Pantanal (MS) | 136 (100) | 126 (92.9) | 97 (71.4) | Leuchtenberger et al. (2020) |
| Corixo Espírito Santo (MT) | 29 (95.3) | 25 (80.6) | 12 (38.7) | Present study |
In the inter-study comparison, characiforms were commonly found in all eight study areas, being present more than 77 percent of the spraints analyzed. Perciforms were also common in the present study, and at Xixuaú Creek, Balbina reservoir, Jaú National Park and in the Corumbá Pantanal, although this order was relatively uncommon in the samples from the Aquidauana River and Juruá River. The differences among sites were highly significant (G test 3 x 8 contingency table; G = 191.5082; P<0.0001). The spraints from Baia das Pedras had proportionally more siluriforms than those from all the other study areas (residuals significant at P<0.01). By contrast, Corixo Espirito Santo had a significantly lower proportion of siluriforms than others Pantanal regions (residuals significant at P<0.05).
The chi-square analysis showed that Baía das Pedras Lake and the Corixo Espírito Santo are significantly distinct to Characiformes and Siluriformes order (χ2 = 17.1301; P<0.0001). We also observed statistically significant findings on the relationship among the order of fish and other studies in the Pantanal region (χ2 = 24.4962; P<0.0001). In the integrated data analysis for all studies in the Amazon and Pantanal regions, there was variation in frequency of occurrence of taxonomic orders with highly significant chi-square value (χ2 = 174.4825; P<0.0001).
DISCUSSION
The results of the present study on two floodplain systems of Pantanal (lake and river branch) indicate that fish is the main source of food for the giant otter, as reported in the previous study of Carter and Rosas (1997), Silva et al. (2013) and Leuchtenberger et al. (2020). The analyses also confirmed the occasional predation of crustaceans and molluscs (Rosas et al., 2008; Silva et al., 2013; Leuchtenberger et al., 2020).
As in previous studies (Duplaix, 1980; Schweizer, 1992; Rosas et al., 1999; Cabral et al., 2010; Silva et al., 2013; Leuchtenberger et al., 2020), the findings of the present study indicate that the diet of the P. brasiliensis populations is composed primarily of fish belonging to the orders Characiformes, Perciformes, and Siluriformes. Cichlids were the principal category of prey at Baía das Pedras, while erythrinids predominated at Corixo Espírito Santo. Previous studies have also emphasized the importance of the erythrinid genus Hoplias (Duplaix, 1980; Laidler, 1984; Schweizer, 1992; Cabral et al., 2010; Rosas-Ribeiro et al., 2012; Silva et al., 2013; Becerra-Cardona et al., 2015; Trujillo et al., 2015, Leuchtenberger et al., 2020) and cichlids (Laidler, 1984; Rosas et al., 1999; Cabral et al., 2010; Silva et al., 2013; Trujillo et al., 2015, Leuchtenberger et al., 2020) in the diet of the giant otter.
The principal difference in the prey categories between Baía das Pedras and Corixo Espírito Santo was the greater frequency of the siluriform families Auchenipteridae, Callicthyidae, Doradidae and Loricariidae in the former environment. Siluriforms are nocturnal and seek refuge on the bottom or in crevices during the day (Kirchheim and Goulart, 2010), although Britski et al. (1999) found that many species are active during the day, especially in turbid waters.
The turbidity of the water has differential effects on the abundance of different fish groups, with the abundance of siluriforms and gymnotiforms increasing in more turbid waters, whereas characiforms and cichlids are more common in more transparent water (Castro et al., 2018). Fish species with sensorial adaptions for conditions of reduced luminosity will tend to predominate in more turbid waters, while visually-oriented species will be more dominant in clearer water, which benefit their foraging strategies (Rodríguez and Lewis Jr., 1997; Kirchheim and Goulart, 2010). At Baía das Pedras, the more restricted lacustrine environment is associated with the accumulation of feces and decomposition of organic matter derived from the high density of fish, waterbirds, and caiman, Caiman yacare (Daudin, 1802) (Nogueira et al., 2002), what connected to an exacerbated decline in the level of the water over the course of the dry season, contribute to the turbidity of the aquatic environment and may be driven the differences observed in the giant otter diet between the different floodplain systems.
Although relatively common in the Pantanal, callichthyids were not consumed frequently at Corixo Espírito Santo (12.4%). On the other hand, this group of fish has been found in 50% of the spraints collected from Corumbá Pantanal (Leuchtenberger et al., 2020) and was the second most common fish family from Baía das Pedras (97.7%). The different habitat characteristics probably influenced the occurrence of callicthyids between Corixo Espírito Santo and Baía das Pedras. However, it is difficult to determine, from the fecal analyses, to what extent feeding preferences or specializations, or the relative availability of prey, influence the composition of the giant otter diet (Rosas et al., 1999), in particular considering that callicthyids are much less common (Rosas-Ribeiro et al., 2012; Silva et al., 2013) or even absent (Cabral et al., 2010) from the giant otter diet in some Amazonian environments. The sedentary and slow swimming behavior of the callicthyids (Britski et al., 1999), associated with the unique rocky substrates of the Baía das Pedras site (Nogueira et al. 2002), may facilitate the capture of these fish at this site, in contrast with the conditions found at the Corixo Espírito Santo. Overall, then, the relative importance of callicthyids in the diet of the giant otters in the present study, in comparison with other localities, may reflect the greater availability of these fish in the shallower parts of the Baía das Pedras, a scenario reinforced by the limnological intensification of the water turbidity during the dry season. More detailed studies will be required to determine the depletion of the availability of other prey taxa in this habitat.
The tendency for an increase in the consumption of loricariid catfish with the intensification of the dry season at Baía das Pedras, may be related to the predominance in this lake of limnophilic taxa, which are tolerant of low oxygen concentrations, and are forced to retreat into remnant pools as the water level decreases during the dry season (Junk et al., 2006). It is important to note that, during the dry season, isolated habitats become increasingly subject to distinct selective pressures that exacerbate the heterogeneity of the remaining aquatic habitats (Thomaz et al., 2007). The morphological differences found in distinct fish species permit their differential exploitation of both feeding resources and physical habitats, which permits the coexistence of the different species (Agostinho et al., 2007; Shibatta et al., 2007). The fish species composition may also vary among the different phases of the flood pulse, depending on the duration and intensity of the hydrological cycle (Alho et al., 2008).
Acknowledgements: The authors are grateful to the researchers Dr. Mário de Pinna of the Ichthyology Sector at the USP Zoology Museum, Dr. Mauro Triques of the Ichthyology Laboratory at UFMG, Dr. Francisco Langeani Neto of the Ichthyology Laboratory at UNESP and Dr. Rosenil Oliveira of the vertebrate collection at the UFMT Biosciences Institute, who provided invaluable input for the fish taxa. We are also grateful to Dr. Adriano Chiarello for his contributions to the manuscript. The authors are also grateful to the Brazilian funding agencies, the Coordination for Higher Education Personnel Training (CAPES), for a graduate stipend, the National Council for Scientific and Technological Development (CNPq), PELD/CNPq site 12 Pantanal Norte Project for funding the present study and the Natural Heritage Private Reserve of SESC Pantanal (RPPN SESC Pantanal) for logistics support and fieldwork related research. The authors wish to express particular thanks to Assis Rondon for invaluable contribution in the field and to Dr. João Batista de Pinho (PPG-ECB/UFMT) and Cristina Cuiabália Neves (SESC Pantanal) for news information about giant otters.
REFERENCES
Agostinho, A.A., Pelicice, F.M., Petry, A.C., Gomes, L.C., Júlio Jr., H.F. (2007). Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat. Ecosyst. Health. 10(2): 174–186
Alho, C.J.R. (2008). Biodiversity of the Pantanal: response to seasonal flooding regime and to environmental degradation. Braz. J. Biol. 68(4, Suppl.): 957–966.
Alho, C.J.R., Sabino, J. (2012). Seasonal Pantanal Flood Pulse: Implications for Biodiversity Conservation – A Review. Oecol. Aust. 16(4): 958–978.
Alho, C.J.R., Mamede, S.B., Benites, M., Andrade, B.S., Sepúlveda, J.J.O. (2019). Ameaças à Biodiversidade do Pantanal Brasileiro pelo Uso e Ocupação da Terra. Ambient. Soc., 22: e01891.
Antas, P.T.Z., Oliveira, L.F.B., Pádua, M.T.J., Pereira, N.C., Vanutky, W.W. (2011). Plano de Manejo da Reserva Particular do Patrimônio Natural do SESC Pantanal (Conhecendo o Pantanal 3) / Brandão, L.G. (Coordenação); 2. ed. Rio de Janeiro : SESC, Departamento Nacional, 2011. 148 p.
Antas, P.T.Z.; Carrara, L.A.; Ubaid, F.K.; Oliveira Júnior, S.B. & Ferreira, L.P. (2016). Aves coloniais das praias da Reserva Particular Natural SESC Pantanal. (Conhecendo o Pantanal 10). Rio de Janeiro: SESC, Departamento Nacional, 2016. 236p.
Ayres, M., Ayres Jr., M., Ayres, D.L., Santos, A.A.S. (2007). BioEstat 5.0: Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas. Sociedade Civil Mamirauá; Belém/PA, Brasil. 364p.
Baginski, L.J., Florentino, A.C., Fernandes, I.M., Penha, J.M.F., Mateus, L.A.F.M. (2007). A dimensão espacial e temporal da diversidade de peixes da zona litoral vegetada de lagoas marginais da planície de inundação do rio Cuiabá, Pantanal, Brasil. Biota Neotrop. 7(3): 233–238.
Becerra-Cardona, M.P., Mallea-Cárdenas, H.A. and Van Damme, P.A. (2015). The use of premaxillary bones of six fish species in giant otter (Pteronura brasiliensis) diet analysis. LATAM 10(2): 131–142.
Britski, H.A. Keve, Z.S.S., Lopes, B.S. (1999). Peixes do Pantanal. Manual de Identificação. Brasília/DF: Embrapa-SPI; Corumbá/MS: Embrapa-CPAP. 184p.
Cabral, M.M.M., Zuanon, J., Mattos, G.E., Rosas, F.C.W. (2010). Feeding habits of Giant Otters Pteronura brasiliensis (Carnívora: Mustelidae) in the Balbina Hydroelectric Reservoir, Central Brazilian Amazon. Zoologia 27(1): 47–53.
Camilo-Alves, C. & Desbiez, A. (2005) The Use Of A Natural Cave For Breeding By Giant Otters In The Brazilian Pantanal: Observations And New Insights On Giant Otter Behavior. IUCN Otter Spec. Group Bull. 22(1): 21 - 24.
Carter, S.K., Rosas, F.C.W. (1997). Biology and conservation of the giant otter, Pteronura brasiliensis. Mammal Rev. 27(1): 1–26.
Carter, S.K., Rosas, F.C.W., Cooper, A.B., Cordeiro-Duarte, A.C. (1999). Consumption rate, food preferences and transit time of captive giant otters Pteronura brasiliensis: Implications for the study of wild populations. Aquat. Mamm. 25(2): 79–90.
Castro, R.J., Henry, R., Ferragut, C., Casartelli, M. (2018). Comparing lacustrine environments: the importance of the kind of habitat on the structure of fishes. Acta Limnol. Bras. 30: e303.
Catella, A.C., Mascarenhas, R.O., Albuquerque, S.P., Albuquerque, F.F., Theodoro, E.R.M. (2008). Sistemas de estatísticas pesqueiras no Pantanal, Brasil: aspectos técnicos e políticos. Panam. J. Aquat. Sci. 3(3): 174–192.
Colman, C.B., Oliveira, P.T.S., Almagro, A., Soares-Filho, B.S., Rodrigues (2019). Effects of Climate and Land-Cover Changes on Soil Erosion in Brazilian Pantanal. Sustainability, 11: 7053.
Duplaix, N. (1980). Observations on the ecology and behaviour of the giant river otter Pteronura brasiliensis in Suriname. Rev. Ecol. - Terre Vie 34: 495–620.
Duplaix, N., Waldemarin, H.F., Groenekijk, J., Evangelista, E., Munis, M., Valesco, V., Botello, J.C. (2008). Pteronura brasiliensis. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. <www.iucnredlist.org>.DOI https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T18711A21938411.en.Downloaded on 25 May 2012.
Duplaix, N., Evangelista, E., Rosas, F.C.W. (2015). Advances in the study of giant otter (Pteronura brasiliensis) ecology, behavior, and conservation: a review. LAJAM 10(2): 75–98.
Fernandes, A.H.B.M., Catella, A.C.,Soriano, B.M.A., Urbanetz, C., Cardoso, E.L., Fernandes, F.A., Bergier, I., Comastri, J.A., Salis, S.M., Tomas, W.M. (2019). BIOMA PANTANAL: oportunidades e desafios de pesquisa para o desenvolvimento sustentável. In: Vilela, E.F., Callegaro, G.M., Fernandes, G.W. Biomas e agricultura. Edition: 1, Chapter 6. Publisher: Vertente Edições pp. 99-121.
FIOCRUZ (2020). Incêndios Florestais no Pantanal 2020. Fundação Oswaldo Cruz (Ministério da Saúde) Nota Técnica 01: 1– 11. (Accessed: 16 Feb 21)
https://agencia.fiocruz.br/sites/agencia.fiocruz.br/files/u34/nt_01_pantanal_final1.pdf Downloaded 1 September 2021
Fonseca, F.R.D., Malm, O., Waldemarin (2005). Mercury levels in tissues of Giant otters (Pteronura brasiliensis) from the Rio Negro, Pantanal, Brazil. Environ. Res. 98: 368–371.
Garcia, D.M., Marmontel, M., Rosas, F.C.W., Santos, F.R. (2007). Conservation genetics of the giant otter (Pteronura brasiliensis) (Zimmermann, 1780) (Carnivora, Mustelidae). Braz. J. Biol. 67(4, Suppl.): 819–827.
Gómez, J.R., Jorgenson, J.P. (1999). An Overview to the Giant Otter – Fisherman Problem in the Orinoco Basin of Colômbia. IUCN Otter Spec. Group Bull. 16(2): 90–96.
Groenendijk, J., Duplaix, N., Marmontel, M., Van Damme, P. & Schenck, C. (2015). Pteronura brasiliensis. The IUCN Red List of Threatened Species 2015: e.T18711A21938411. https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T18711A21938411.en Downloaded on 02 September 2021.
INPE (2020). Monitoramento dos Focos Ativos por Bioma (Queimadas). Programa Queimadas – Instituto Nacional de Pesquisas Espaciais. (Accessed: 16 Feb 21) http://queimadas.dgi.inpe.br/queimadas/portal-static/estatisticas_estados.
Ivory, S.J., McGlue, M.M., Spera, S., Silva, A., Bergier, I. (2019). Vegetation, rainfall, and pulsing hydrology in the Pantanal, the world’s largest tropical wetland. Environ. Res. Lett. 14: 124017.
Junk, W.J., Cunha, C.N., Wantzen, K.M., Petermann, P., Strüssmann, C., Marques, M.I., Adis, J. (2006). Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 68: 278–309.
Kirchheim, P.D.; Goulart, E. (2010). Ecomorfologia de Predação e Antipredação em Siluriformes (Osteichthyes). Oecol. Aust. 14(2): 550–568.
Laidler, P.E. (1984). The Behavioural Ecology of the Giant Otter in Guyana. Dissertation for the Degree of Doctor of Philosophy. University of Cambridge. 296p.
Leite, G.F.M., Rezende, R.S., Silva, H.P., Muniz, C.C. (2018). Effects of Flood Pulse on the Community of Loricariidae (Pisces, Siluriformes) in Oxbow Lakes of the Pantanal, Brazil. Bol. Inst. Pesca 44(1): 35–43.
Leuchtenberger, C., Mourão, G. (2008). Social Organization and Territoriality of Giant Otters (Carnivora: Mustelidae) in a Seasonally Flooded Savana in Brazil. Sociobiology 52(2): 257–270.
Leuchtenberger, C., Oliveira-Santos, G.R., Magnusson, W., Mourão, G. (2013). Space use by giant otter groups in the Brazilian Pantanal. J. Mammal. 94(2): 320–330.
Leuchtenberger, C., Rheigantz, M.L., Zucco, C.A., Catella, A.C., Magnusson W.E., Mourão, G. (2020). Giant otter diet differs between habitats and from fisheries offtake in a large Neotropical floodplain. J. Mammal., 101(6):1650–1659.
Magalhães, C. (2000). Caracterização da comunidade de crustáceos Decápodos do Pantanal. Mato Grosso do Sul. RAP, 2000. P. 175–182 (Boletim de Avaliação Biológicas).
MMA (2018). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume II – Mamíferos / 1. ed. Brasília, DF : ICMBio/MMA (Ministério do Meio Ambiente), 2018. (622 p.)
Mateus, L.A.F., Penha, J.M.F., Petrere, M. (2004). Fishing resources in the rio Cuiabá basin, Pantanal do Mato Grosso, Brazil. Neotrop. Ichthyol., 2(4): 217–227.
Moura, N.A., Val, A.L. (2019). Migração Lateral de Peixes e a Vulnerabilidade da Baía do Chacororé. Pantanal de Barão de Melgaço, Mato Grosso, Brasil. Rev. Biol. Neotrop. / J. Neotrop. Biol. 16(1): 1–8.
Nogueira, F., Silva, R.L. Silva, A.J. Souza, M.D., Bachega, I. (2002). Seasonal and diel limnological differences in a tropical floodplain lake (Pantanal of Mato Grosso, Brazil). Acta Limnol. Bras. 14(3): 17–25.
Nunes da Cunha, C., Junk, W.J. (2004). Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Appl. Veg. Sci. 7: 103–110.
Paglia, A.P., Fonseca, G.A.B., Rylands, A.B., Herrmann, G., Aguiar, L.M.S., Chiarello, A.G., Leite, Y.L.R., Costa, L.P., Siciliano, S., Kierulff, M.C.M., Mendes, S.L., Tavares, V.C., Mittermeier, R.A., Patton, J.L. (2012). Lista Anotada dos Mamíferos do Brasil / Annotated Checklist of Brazilian Mammals. 2ª Edição / 2nd Edition. Occasional Papers in Conservation Biology, No. 6. Conservation International, Arlington, VA. 76p.
Pains da Silva, H., Petry, A.C., da Silva, C.J. (2010). Fish communities of the Pantanal wetland in Brazil: evaluating the effects of the upper Paraguay river flood pulse on baı´a Caic¸ara fish fauna. Aquat Ecol. 44: 275–288.
Pickles, R.S.A., Groombridge, J.J., Zambrana-Rojas, V.D., Van Damme, P., Gottelli, D., Ariani, C.V., Jordan, W.C. (2012). Genetic diversity and population structure in the endangered giant otter, Pteronura brasiliensis. Conserv. Genet. 13: 235–245.
Rodriguez, M.A., Lewis Junior, W.M. (1997). Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco River. Ecol. Monogr. 67(1): 109–128.
Rosas, F.C.W., Zuanon, J.A.S., Carter, S.K. (1999). Feeding Ecology of the Giant Otter Pteronura brasiliensis. Biotropica 31(3): 502–506.
Rosas, F.C.W., Waldemarin, H.F., de Mattos, G.E. (2008). Ariranha, Pteronura brasiliensis (Zimmermann, 1780). In: Machado, A.B.M., Drummond, G.M. and Paglia, A.P. (Eds). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Belo Horizonte, MMA, Fundação Biodiversitas, p. 800–801. 1420p.
Rosas-Ribeiro, P.F., Rosas, F.C.W., Zuanon, J.A.S. (2012). Conflict between fishermen and giant otters Pteronura brasiliensis in Western Brazilian Amazon. Biotropica. 44: 437–444.
Schulz, C., Whitney, B.S., Rosseto, O.C., Neves, D.M., Crabb, L., Oliveira, E.C., Lima, P.L.T., Afzal, M., Laing, A.F., Fernandes, L.C.S., Silva, C.A., Steinke, V.A., Steinke, E.T., Saito, C.H. (2019). Physical, ecological and human dimensions of environmental change in Brazil's Pantanal wetland: Synthesis and research agenda. Sci. Total Environ. 687: 1011–1027.
Schweizer, J. (1992). Ariranhas no Pantanal. Ecologia e Comportamento da Pteronura brasiliensis. Editora Brasil Natureza (EDIBRAN), Curitiba, Brasil. 200p.
Shibatta, O.A., Gealh, A.M., Bennemann, S.T. (2007). Ichthyofauna from the middle and upper stretches of rio Tibagi basin, Paraná, Brazil. Biota Neotrop. 7(2): 125–134.
Shimabukuro, Y.E., Dutra, A.C., Arai, E., Duarte, V., Cassol, H.L.G., Pereira, G., Cardozo, F.S. (2020). Mapping Burned Areas of Mato Grosso State Brazilian Amazon Using Multisensor Datasets. Remote Sens. 12: 3827.
Silva, R.E., Rosas, F.C.W., Zuanon, J. (2013). Feeding ecology of the giant otter (Pteronura brasiliensis) and the Neotropical otter (Lontra longicaudis) in Jaú National Park, Amazon, Brazil, J. Nat. Hist. 48(7-8): 465–479.
Silva, J.S.V., Abdon, M.M. (1998). Delimitação do Pantanal Brasileiro e suas sub-regiões. Pesqui. Agropecu. Bras. 33: 1703–1711.
Silva-Junior, C.A., Teodoro, P.E., Delgado, R.C., Teodoro, L.P.R., Lima, M., Pantaleão, A.A., Baio, F.H.R., Azevedo, G.B., Azevedo, G.T.O.S., Capristo-Silva, G.F., Arvor, D., Facco, C.U. (2020). Persistent fire foci in all biomes undermine the Paris Agreement. in Brazil. Sci Rep 10: 16246.
Tejerina-Garro, F.L.,; Fortin, R., Rodriguez, M.A. (1998). Fish Community structure in relation to environmental variation in floodplain lakes of the Araguaia river, Amazon Basin. Env. Biol. Fishes 51(4): 399–410.
Thielen, D, Schuchmann, K-L., Ramoni-Perazzi, P., Marquez, M., Rojas, W., Quintero, J.I., Marques, M.I. (2020). Quo vadis Pantanal? Expected precipitation extremes and drought dynamics from changing sea surface temperature. PLoS ONE 15(1): e0227437.https://doi.org/10.1371/journal.pone.0227437
Thomaz, S.M., Bini, L.M., Bozelli, R.L. (2007). Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13
Trujillo, F., Caro, A., Martínez, S., Rodríguez-Maldonado, M.V. (2015). Negative interactions between giant otters (Pteronura brasiliensis) and local fisheries in the Amazon and Orinoco basins in Colombia. LATAM 10(2): 122–130.
Zar, J.H. (1996). Biostatistical Analysis. 3ª ed. Prentice Hall International. New Jersey.
Résumé: Adaptabilité Alimentaire de la Loutre Géante, Pteronura brasiliensis, (Mammifères: Mustélidés), dans Deux Reseaux de Plaines Inondables de la Zone Humide du Pantanal, dans L’état du Mato Grosso, au Brésil
La loutre géante, Pteronura brasiliensis, est un mammifère presque exclusivement piscivore de la famille des Mustélidés, et une espèce en voie de disparition. La présente étude compare le régime alimentaire de la loutre géante dans deux réseaux de plaines inondables de la zone humide du Pantanal, un lac permanent et un méandre de la rivière, dans les zones humides de Poconé et Barão de Melgaço dans l'État brésilien du Mato Grosso. Des échantillons ont été collectés pendant la saison sèche, avec 43 épreintes prélevées dans le lac et 31 dans le méandre de la rivière. Les espèces de poissons présentes dans les échantillons ont été identifiées sur base de la comparaison de fragments d'os trouvés dans les épreintes avec des spécimens de poissons collectés dans les deux réseaux de plaines inondables. Les régimes alimentaires étaient composés principalement de poissons des familles d’Erythrinidés (94,6%), de Cichlidés (91,9%), de Pimelodidés (70,3%), de Callichthyidés (63,5%), et de Doradidés (60,8%). Les poissons-chats (Siluriformes) étaient plus abondants dans les échantillons du lac, tandis que les characins (Characiformes) étaient bien représentés dans le méandre de la rivière. Alors que la famille des Callichthyidés (siluriformes) était une composante importante du régime alimentaire des loutres dans les deux zones d'étude, elle a rarement été signalée dans des études antérieures sur P. brasiliensis dans les régions de l'Amazone ou du Pantanal. La plupart des poissons signalés dans le régime alimentaire de la loutre ne sont pas ciblés par les pêcheries commerciales du Mato Grosso. Les caractéristiques variables des différents systèmes aquatiques sont des déterminants importants de la composition de la faune piscicole locale et sont donc pertinentes pour l'écologie alimentaire de la loutre géante dans la zone humide du Pantanal.
Revenez au dessus
Resumen: Adaptibilidad Dietaria de la Nutria Gigante, Pteronura brasiliensis (Mammalia: Mustelidae), en Dos Sistemas de Planicies de Inundación en el Pantanal, Estado de Matogrosso, Brasil
La Nutria Gigante, Pteronura brasiliensis, es un mamífero casi exclusivamente piscívoro de la Familia Mustelidae, y una especie en peligro de extinción. El presente estudio comparó la dieta de las nutrias gigantes en dos sistemas de planicies inundables del Pantanal, un lago permanente y un brazo del rio, en los humedales Poconé y Barão de Melgaço, en el estado brasileño de Mato Grosso. Las muestras se recolectaron durante las estaciones secas, con 43 heces recolectadas del lago y 31 del brazo del río. Las especies de peces en las muestras de heces se identificaron en base a la comparación de fragmentos de huesos con los especímenes de peces recolectados en los dos sistemas de planicies de inundación. Las dietas estuvieron compuestas principalmente por peces de la familia Erythrinidae (94.6%), Cichlidae (91.9%), Pimelodidae (70.3%), Callichthyidae (63.5%) y Doradidae (60.8%). Los bagres (Siluriformes) fueron más abundantes en las muestras del lago, mientras que los Characiformes estaban bien representados en el brazo del río. Si bien la família Callichthyidae fue un componente destacado de la dieta de las nutrias gigantes en las dos áreas de estudio, rara vez se había registrado en estudios previos de P. brasiliensis en las regiones de la Amazonia o del Pantanal. La mayoría de los peces registrados en la dieta no son de interés de las pesquerías comerciales en Mato Grosso. Las distintas características de los diferentes sistemas acuáticos son importantes determinantes de la composición de la fauna íctica local y, por lo tanto, son relevantes para la ecología alimentaria de las nutrias gigantes en el Pantanal.
Vuelva a la tapa
Resumo: Adaptabilidade na Dieta de Ariranhas, Pteronura brasiliensis (Mammalia: Mustelidae), em Dois Sistemas da Planície de Inundação do Pantanal, Mato Grosso, Brasil
A ariranha, Pteronura brasiliensis, é um mamífero quase exclusivamente piscívoro da Família Mustelidae e uma espécie em perigo de extinção. O presente estudo comparou a dieta das ariranhas em dois sistemas hídricos da planície inundável do Pantanal, uma lagoa permanente (baía) e um canal de rio (corixo), nos pantanais de Poconé e Barão de Melgaço, no estado de Mato Grosso. As amostras foram coletadas durante a estação de seca, com 43 fezes sendo registradas na baía e 31 no corixo. As espécies de peixes nos restos fecais foram identificadas com base na comparação de fragmentos ósseos dos espécimes de peixes coletados nos sistemas pantaneiros. As dietas foram representadas principalmente por peixes das famílias Erythrinidae (94.6%), Cichlidae (91.9%), Pimelodidae (70.3%), Callichthyidae (63.5%) e Doradidae (60.8%). Os Siluriformes foram mais abundantes nas amostras da baía, enquanto os Characiformes mais representados no corixo. A família Callichthyidae foi um item importante na dieta das ariranhas nas áreas de estudo, especialmente na baía, apesar de raramente ser registrada em outros estudos na região Amazônica e do Pantanal. A maioria dos peixes registrados na dieta das ariranhas não é alvo da pesca comercial no Mato Grosso. As distintas características dos sistemas aquáticos são importantes determinantes da composição da ictiofauna local e, portanto, relevantes para ecologia alimentar das ariranhas na planície do Pantanal.
Voltar ao topo


