IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 38 Issue 4 (August 2021)
Citation: Talegaonkar, R., Salaria, S., Bhargava, D., Jena, J., Rahul, S.K., Dhamorikar, A. and Chanchani, P. (2021). Habitat Use by the Eurasian Otter (Lutra lutra Linnaeus 1758) in a Non-Protected Area of Madhya Pradesh, India. IUCN Otter Spec. Group Bull. 38 (4): 217 - 227
Habitat Use by the Eurasian Otter (Lutra lutra Linnaeus 1758) in a Non-Protected Area of Madhya Pradesh, India
Rahul Talegaonkar1*, Saloni Salaria1, Dhirendra Bhargava2, Jyotirmay Jena1, Snehit Kumar Rahul1, Aniruddha Dhamorikar1, and Pranav Chanchani1
1WWF-India, 172 B Lodi Estate, New Delhi 110 003, India
2Madhya Pradesh Forest Department, Bhopal 462 004, India
*Corresponding Author: rtalegaonkar@wwfindia.net
(Received 24th November 2020, accepted 25rd February 2021)
Abstract: Eurasian otter is one of the three otters found in India. In Central India, it was recently photo-captured in Balaghat district. We studied habitat use of Eurasian otters (Lutra lutra) by sampling 57 stream segments along the Wainganga River and Uskal stream in a reserve (non-protected) forest of Balaghat Forest Division in the months of March-April 2018 using sign surveys. We used an occupancy-based approach to determine the influence of habitat covariates on otter occupancy. Bank substrate had a significant positive impact on detection probability of otters. The probability of habitat use by otters strongly decreased as the bank width increased. Future studies should focus to better understand the impact of human activities on the distribution, demography, and behaviour of otters
Keywords: Lutra lutra, habitat use modeling, Central India, riverine ecosystem
INTRODUCTION
India is home to three species of otters - the Smooth-coated (Lutrogale perspicillata), the Asian small-clawed (Aonyx cinerea), and the Eurasian otter (Lutra lutra). The Smooth-coated otter is distributed throughout the country, but the other two were known to be restricted to the Himalayas, north of the Ganges and southern India. They were considered to be absent from Central India (Pocock, 1939; Hussain and Choudhury, 1997; Prater, 1971; Foster-Turley and Santiapillai, 1990; Hussain, 1993, 2012), but the Eurasian otter was historically present (Low, 1907). It was recently reported in Satpuda Tiger Reserve (Joshi et al., 2016) and Balaghat Forest Circle (Jena et al., 2016) in Madhya Pradesh. Also, the Asian small-clawed otter was reported in Odisha, Eastern India (Mohapatra et al., 2014). Otters are primarily threatened by habitat loss through resource exploitation such as sand mining, overfishing, as well as infrastructure development including river damming, bank concretization, and diversion (Roos et al., 2015).
Otters are adapted for a semiaquatic life (Pocock, 1939; Prater, 1971) and are a shy and elusive species. They are usually found along rivers, streams, hill creeks, and wetlands with adequate bank-side vegetation, and require cavities among tree roots, pile of rocks, wood and debris, and holes in riverbank for shelter and breeding (Menon, 2009; Hussain, 2012). In Scotland, Kruuk et al. (1993) observed that otters preferred streams with higher fish biomass and that the fish biomass decreased exponentially with the stream width.
The Eurasian otter is a large otter with a coarse, dusky brown coat that looks shaggy when dry and bedraggled when wet, but never as smooth as the smooth-coated otter (Menon, 2009). It often bears spots on its lips and nose, and the rhinarium is naked and black, with a W-shaped upper margin. The tail is conical and long, over half the head and body length. The five toes have strong claws and webbing extending to the end of the digit (ibid). The Eurasian otter has been well-studied in Europe (Chanin, 1985; Mason and Macdonald, 1986), but its ecology in Asia lacks detailed studies (Foster-Turley and Santiapillai, 1990). Here we study the drivers that influence the occurrence of Eurasian otter in a riverine ecosystem amidst a mixed-deciduous and sal (Shorea robusta) dominated forest of Central India, in the state of Madhya Pradesh. We predict that (i) wider river banks are likely associated with lower otter habitat use as they are easily accessible to predators and also provide less complex habitats for denning; (ii) higher standard deviation of river width is likely associated with high otter use as it provides greater habitat complexity; (iii) and the presence of human activities such as fishing and sand mining negatively affect the otter habitat use.
MATERIAL AND METHODS
Study Area
Our study area includes four forest ranges of North and South Balaghat Forest Divisions – North Lamta, South Lamta, Logour, and Balaghat (Figure 1). The area is approximately 963 km2 and is characterized by mixed dry deciduous and sal-dominated forests. Several perennial streams, including Uskal, a major tributary of the Wainganga, flows through it. The Wainganga River flows to North to South, with the Uskal stream joining the Wainganga in South Lamta Range. Two reservoirs, one built on the Wainganga River in Dhuti, and another in Gangulpara, supplies water for small-scale non-monsoonal farming. The landscape mainly comprises hills, tablelands and plains, and serves as a connecting corridor between Kanha and Pench tiger reserves. The highest point is at 818 m asl in Balaghat Range and decreases gradually at 298 m asl towards west. According to the 2018 All India Tiger Estimation (Jhala et.al., 2020) 21 tigers were identified in North and South Forest Divisions.
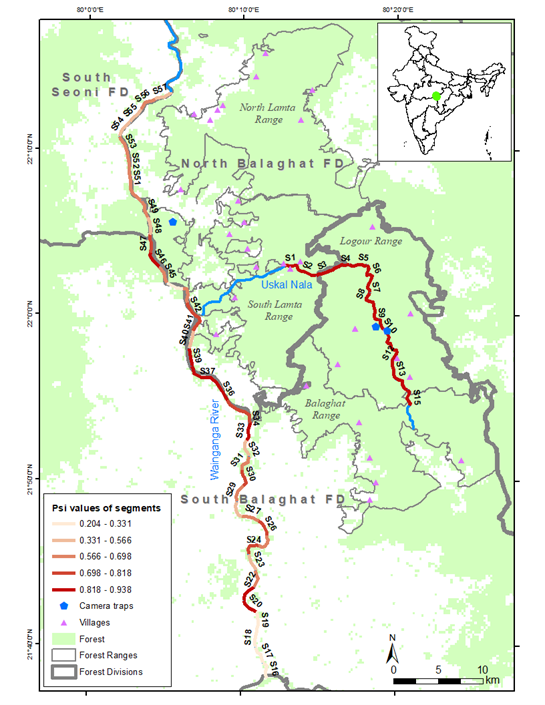
There are 35 villages within four ranges surveyed, with a density of 13.4 persons per km2. Since this is a territorial forest area primarily managed for timber extraction and forestry operations, livestock grazing is permitted in areas where active forestry management is not being undertaken, and collection of dry wood as fuel and NTFP is permitted.
Methodology
We conducted sign surveys along 45 km of the Uskal stream and 75 km of the Wainganga river during March-April 2018 (Figure 1). We identified the species and the survey areas based on the camera traps deployed during the study for large carnivores in Balaghat, and information from the locals and the Madhya Pradesh Forest Department. We defined our sampling units by 57 two-kilometre-long sections along these two drainages based on previous studies by Erlinge (1968) and Kruuk (1995). Replication was spatial, rather than temporal, with detection (1) – non detection (0) data being collected along 400 m segments within each of the 57 sampling units (Perinchery et al., 2011; Charbonnel et al., 2014). Surveys were conducted between 0800 hrs and 1200 hrs. Both sides of the banks were thoroughly searched on foot to record signs of spraints, pugmarks, and holts (otter dens) by the same group of observers for all the surveys. In addition to data on otter sign detection, information was collected on six individual covariates - river width, bank width, presence/indication of fishing and sand mining, bank substrate, and bank vegetation - to assess their impact on the otter habitat use (Table 1). We checked for multicollinearity and found no correlation between our covariates
Given that data collected along linear features like streams are likely to be spatially autocorrelated (species use of one segment is not independent of use of previous segments), we analyzed our data using a model that accounts for spatial clustering of the response variable, following Hines et al. (2010) model in program MARK (ver. 9.0, White and Burnham, 1999). This model has four parameters: Ψ (psi) - the probability that the segment is occupied by the species, p - the probability of detecting the species, and θ and θ’ - the probabilities of habitat use of a segment given the non-use and use of the previous segment. In our analysis, we used a two-step approach to model parameters of interest. We first began by modelling the effect of bank substrate, bank width and bank cover on detection probability p, while retaining a global covariate structure for Ψ (Bank width+River/ stream width+Fishing+Sand mining) (Table 2). Then using the best supported model structure for p, we assessed the influence of covariates on habitat use Ψ (Table 3). Model support was evaluated using AIC (Burnham and Anderson, 2002).
RESULTS
We surveyed a total of 120 km of riverbank edges along Uskal and Wainganga. We detected otter signs in 35 of the 57 sampling units and 108 of the 285 (400 m) segments. The naïve estimate of otter occurrence was 0.61.
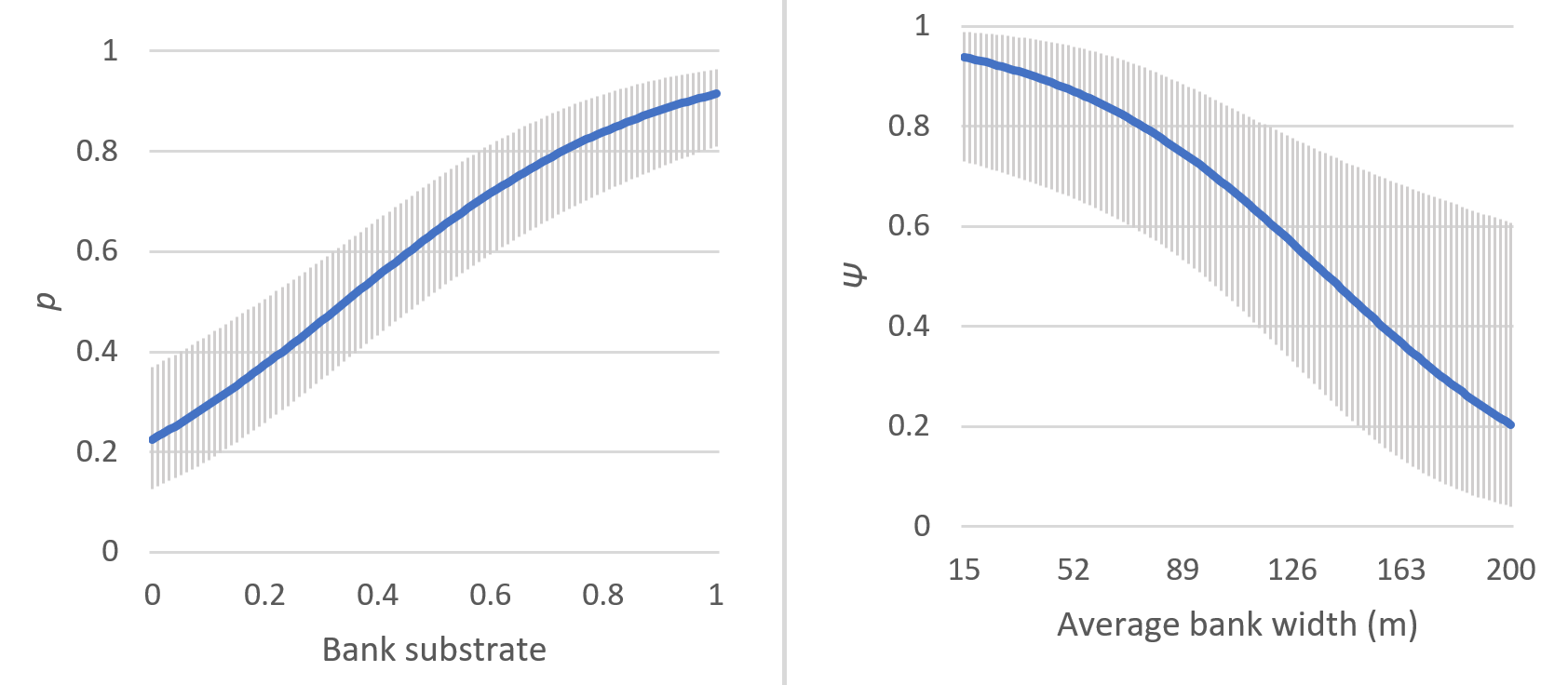
Our analysis to determine the effect of bank substrate, bank width, and bank cover on detection probability revealed that only bank substrate had a significant positive impact on detection probability of otters (Table 2, Figure 2a). The detection was much better in the sandy and muddy substratum.
Given that model weights were distributed across several models for Ψ; we report model averaged estimates for all parameters. The model averaged estimate for Ψ was 0.77 ± 0.11 (CI 0.49 - 0.92)). We found evidence for strong spatial autocorrelation in segment-level otter presence. Otter occurrence probability on segments was low 0.25 ± 0.30 (CI 0.02 - 0.88) when the previous segment was not used θ, and several orders of magnitude higher when the previous segment was also used (θ’ = 0.98 ± 0.03 (CI 0.74 - 0.99)) and p was 0.57 ± 0.06 (CI 0.45 - 0.68)).
Among the various models for estimating habitat use, the best model included bank width (Table 3). Estimates of β indicated that the probability of use by otters strongly decreased as the bank width increased (Figure 2b). The estimated Ψ values of the sampled segments are reported in Table 4. Other than bank width, covariates in the model did not have significant influence on otter habitat use.
DISCUSSION
Eurasian otters prefer upstream habitat along rivers, hill creeks and streams with adequate bank-side vegetation as shelter and to breed (Menon, 2009; Hussain, 2012). Most of their behavioural observations are restricted to the Himalaya and the foothills. We show that Eurasian otters in Central India also prefer similar habitats to feed, rest, and breed. The first district gazetteer of Balaghat (Low, 1907) considered this species to be “found in all parts of the District in rivers and nalhas”. It is likely that this species has faced a significant decline in populations in Central India, particularly in Balaghat district in the 20th century. Their habitat specificity, elusive nature, and low population has limited their presence to a few drainages within forests, and they are infrequently detected. This species was reported in Central India in 2016 at Satpuda Tiger Reserve (Joshi et al., 2016) and Balaghat (Jena et al., 2016) during camera trapping surveys for large carnivores.
Our surveys aimed at understanding the occurrence of Eurasian otters along two important drainages within Balaghat. While a variety of habitat features and human disturbance covariates were expected to influence otter habitat use, most covariates we used were associated with considerable uncertainty. However, the strong inverse relationship between bank width and otter habitat use is informative. Eurasian otters being shy and elusive animals prefer areas which have narrow bank width, minimizing distance between them and river pools where they forage and meet other life history needs, but importantly where they may also find cover from terrestrial predators. Moreover, areas associated with narrower banks were usually rockier and more rugged, offering more complex habitats that offered cover, while areas with wider banks were sandy and exposed. Our study corroborates the findings of other study on Eurasian otters in India (Hussain, 2012).
Given that Balaghat Forest Divisions are a Reserve Forest, it is subjected to several management practices such as selective felling. It also accommodates fuel, fodder and forest produce needs of various local communities. Fishing for household consumption, livestock grazing, and small-scale sand extraction for household use are all permitted in certain areas. We did not find any discernible relationships between local fishing and local sand mining on otter habitat use, possibly because these activities are dispersed, occur at low intensities and were measured imperfectly by using simple indices. Local fishing is non-commercial, restricted to narrow river-ways, ideally in and around rocky areas, which are also the preferred habitats of the Eurasian otters, so more work is needed to understand potential impacts on otter populations, den sites, and also assess the attitudes of fisherfolk towards otters. On the other hand, sand mining is restricted to sandy riverbanks especially where the bank-width is quite broad, and likely has little effect on otter habitat use. Further studies need to be undertaken to better understand the impact of human activities on the distribution, demography and behaviour of otters.
Because Eurasian otter rely on fish as a primary source of food (Hussain, 2012), fishing in the dry season by humans may have impacts that are not evident in other seasons. In some locations of the study area such as the Uskal stream, we were informed of people using ichthyotoxic plants to harvest fish. This type of fishing kills all the fishes in the puddles which are then harvested, suggesting competition for a food source during the dry season. Interactions with local fishing groups revealed that they have observed the otters in areas where they fish, and on occasion the otters have destroyed the fishing nets laid overnight. However, we were informed that the damage is easily repaired and that there is no retaliation by local communities, suggesting that otter presence is tolerated in the region.
We recommend forest rivers be given priority for conservation, the tree cover along rivers exempt from coupe felling, and the critical otter habitats be identified and regularly monitored. We also recommend additional studies on otter behaviour, abundance, and population estimation to develop conservation interventions. Further studies also need to be undertaken to determine the subspecies through genetic sampling from otter spraints. Although humans and otters are sharing the same food source and habitat, understanding the interplay of this interaction in cultural, natural, and historic context is crucial to integrate communities as stewards of otter conservation.
Acknowledgements: The authors would like to thank the Madhya Pradesh Forest Department and the Balaghat Forest Circle for their support and permission to conduct this study. Priya Poonia and Upendra Dubey for helping with the fieldwork. Thanks are also due to Dr. Dipankar Ghose, Dr. Sejal Worah, and Soumen Dey for their support through WWF-India.
REFERENCES
Chanin, P. (1985). The natural history of otters. Croom Helm, London: 1- 179.
Charbonnel, A., D’Amico, F., Besnard, A., Blanc, F., Buisson, L., Némoz, M. and Laffaille, P. (2014). Spatial replicates as an alternative to temporal replicates for occupancy modelling when surveys are based on linear features of the landscape. J. of Applied Ecology, 51(5): 1425-1433. https://doi.org/10.1111/1365-2664.12301
Burnham, K.P., Anderson, D.R. (2002). Model selection and multimodel inference: A practical information-theoretic approach (2nd ed.). Springer, New York.
Erlinge, S. (1968). Territoriality of the Otter Lutra lutra L. Oikos, 19: 81 - 98
Foster-Turley, P., Santiapillai, C. (1990). Action plan for Asian otters. In P. Foster-Turley, Macdonald, M.S., Mason, C.F. (Eds.), Otters: An Action Plan for their Conservation (pp. 52-63). IUCN, Gland, Switzerland.
Harihar, A., Pandav, B. (2012). Influence of connectivity, wild prey and disturbance on occupancy of tigers in the human-dominated western Terai Arc Landscape. PLoS ONE 7(7), e40105. https://doi.org/10.1371/journal.pone.0040105
Hines, J. E., Nichols, J. D., Royle, J. A., Mackenzie, D. I., Gopalaswamy, A., Kumar, S., Karanth, K. (2010). Tigers on trails: occupancy modeling for cluster sampling. Ecological Applications, 20(5): 1456-1466.
Hussain, S.A. (1993). Aspects of ecology of smooth-coated otter (Lutra perspicillata) in National Chambal Sanctuary. [Doctoral dissertation, Aligarh Muslim University]. Shodhganga
Hussain, S.A., Choudhury, B.C. (1997). Status and distribution of smooth-coated otter Lutra perspicillata in National Chambal Sanctuary. Biological Conservation, 80(2): 199-206.
Hussain, S.A. (2012). Mammals of South Asia (A.J.T. Johnsingh, Manjrekar, N. (Eds.). Universities Press, India.
Jena, J., Bhargava, D., Borah, J., Dey, S. (2016). Notes on the Occurrence of the Eurasian Otter (Lutra lutra L.) in the Forest of Balaghat, Madhya Pradesh, India. IUCN Otter Spec. Group Bull, 33(2): 59-63.
Jhala, Y.V., Qureshi, Q., Nayak, A.K.(eds). (2020). Status of tigers, copredators and prey in India, 2018. National Tiger Conservation Authority, Government of India, New Delhi, and Wildlife Institute of India, Dehradun.
Joshi, A.S., Tumsare, V.M., Nagar, A.K., Mishra, A.K., Pariwakam, M. P. (2016). Photographic Records of Eurasian Otter Lutra lutra from the Central Indian Landscape. IUCN Otter Spec. Group Bull. 33(1): 73-78.
Kruuk, H. (1995). Wild otters - Predation and Populations. Oxford University Press
Kruuk, H. (2006). Otters: Ecology, Behavior and Conservation. Oxford University Press.
Kruuk, H., Carss, D. N., Conroy, J. W. H. & Durbin, L. S. (1993). Otter ( Lutra lutra L.) numbers and fish productivity in rivers in north-east Scotland. Zoological Society of London Symposia, 65: 171-191
Low, C.E. (1907). Central Provinces District Gazetteers: Balaghat District. Volume A, Descriptive, 36 pp.
Mason, C.F., Macdonald, S.M. (1986). Otters ecology and conservation. Cambridge: Cambridge University press.
Menon, V. (2009). Indian mammals a field guide. Princeton University Press.
Mohapatra, P., Palei, H.S., Hussain, S.A. (2014). Occurrence of Asian small-clawed otter Aonyx cinereus (Illiger, 1815) in Eastern India. Current Science, 107: 367-370.
Perinchery, A., Jathanna, D., Kumar, A. (2011). Factors determining occupancy and habitat use by Asian small-clawed otters in the Western Ghats, India. Journal of Mammalogy, 92(4): 796-802.
Pocock, R.I. (1939). Notes on some British Indian otters with description of two new species. J. Bombay Nat. Hist. Soc., 41: 514-518.
Prater, S.H. (1971). The book of Indian animals (3rd ed.). Bombay Natural History Society, Bombay.
Roos, A., Loy, A., de Silva, P., Hajkova, P., Zemanová, B. (2015). Lutra lutra. The IUCN Red List of Threatened Species. 2015. https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12419A21935287.en
Royle, J.A., Dorazio, R.M. (2008). Hierarchical modeling and inference in ecology. Academic Press, New York, New York, USA.
White, G.C., and Burnham, K.P. (1999) Program MARK: Survival estimation from populations of marked animals. Bird Study, 46: S120-S139. https://doi.org/10.1080/00063659909477239
Résumé: Utilisation de l‘Habitat par la Loutre Eurasienne (Lutra lutra, Linnaeus, 1758) dans ane Aire non protégée du Madhya Pradesh, en Inde
La loutre eurasienne est l'une des trois loutres que l’on trouve en Inde. Elle a récemment été photographiée en Inde centrale dans le district de Balaghat. Nous avons étudié l'utilisation de l'habitat des loutres eurasiennes (Lutra lutra) à l'aide de relevés d’indices de présence en échantillonnant 57 sections de cours d'eau le long de la rivière Wainganga et du ruisseau Uskal, dans une réserve forestière (non protégée) de la Division Foret de Balaghat, au cours des mois de mars à avril 2018. Nous avons utilisé une approche basée sur l'occupation pour déterminer l'influence des covariables de l'habitat sur la présence de la loutre. Le substrat de la berge a eu un impact positif significatif sur la probabilité de détection des loutres. La probabilité d'utilisation de l'habitat par les loutres diminuait fortement lorsque la largeur de la berge augmentait. Les études futures devraient viser à mieux comprendre l'impact des activités humaines sur la répartition, la démographie et le comportement des loutres..
Revenez au dessus
Resumen: Uso del Hábitat por la Nutria Eurasiática (Lutra lutra Linnaeus 1758) en un Área no protegida de Madhya Pradesh, India
La nutria Eurasiática es una de las tres nutrias que se encuentran en la India. En India Central fue recientemente foto-capturada en el distrito de Balaghat. Estudiamos el uso de hábitat de las Nutrias Eurasiáticas (Lutra lutra) muestreando 57 segmentos a lo largo del Río Wainganga y el arroyo Uskal, en un bosque de reserva (no-protegido) de la División Forestal de Balaghat, en Marzo-Abril de 2018, utilizando relevamiento de signos. Usamos un enfoque basado en la ocupación, para determinar la influencia de las covariables de hábitat en la ocupación por nutrias. El sustrato de las barrancas tuvo un impacto positivo significativo en la probabilidad de detección de las nutrias. La probabilidad de uso de hábitats por las nutrias disminuyó fuertemente a medida que se incrementaba el ancho de las barrancas. Los estudios futuros deberían enfocarse en entender mejor el impacto de las actividades humanas en la distribución, demografía y comportamiento de las nutrias.
Vuelva a la tapa
सारांश: मध्य प्रदेश, भारत के एक गैर-संरक्षित क्षेत्र में यूरेशियन ऊदबिलाव (Lutra lutra Linnaeus 1758) द्वारा पर्यावास का उपयोग
यूरेशियन ऊदबिलाव भारत में पायी जाने वाली तीन ऊदबिलाव की प्रजाति में से एक हैं । हाल ही में मध्य भारत के बालाघाट जिले में एक कैमरा ट्रैप अध्य्यन में इसे दर्ज किया गया है । हमने वर्ष २०१८ के मार्च- अप्रैल महीने में बालाघाट वन मंडल के संरक्षित (गैर -आरक्षित ) क्षेत्र में बैनगंगा एवं उकसल नदी के किनारे ५७ खण्डों में साक्ष्य सर्वेक्षण के माध्यम से यूरेशियन ऊदबिलाव के आवास का अध्ययन किया । हमने ऊदबिलाव के आवास में उसकी स्थिति पर उसके आवास के सहकारकों के प्रभाव का पता लगाने के लिए एक अधिभोग आधारित दृष्टिकोण का उपयोग किया है। नदी के किनारों की भूमि का ऊदबिलाव के खोज की सम्भावना में एक महत्वपूर्ण सकारात्मक प्रभाव हुआ । जैसे जैसे नदी के किनारों की चौड़ाई बढ़ती जाती है वैसे वैसे ऊदबिलाव के आवास की सम्भावना घटती जाती है । भविष्य के अध्ययन को मानवीय गतिविधियों का ऊदबिलाव के वितरण, जनसांख्यिकी और व्यवहार पर प्रभाव को समझने के लिए केंद्रित होना चाहिए।
शीर्ष पर लौटें






