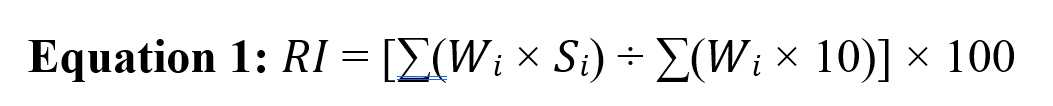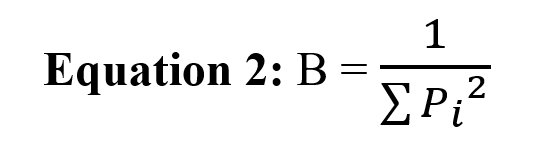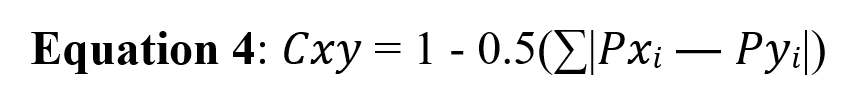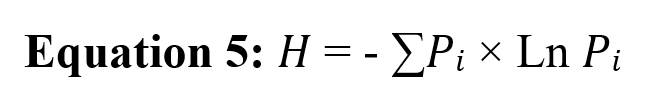IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Volume 41 Issue 5 (December 2024)
Citation: Sojdeh, N., Naderi, S., Mirzajani, A., and Shadloo, S. (2024). Diet of the Eurasian Otter (Lutra lutra) in Boujagh National Park, Guilan, Iran. IUCN Otter Spec. Group Bull. 41 (5): 262 - 279
Diet of the Eurasian Otter (Lutra lutra) in Boujagh National Park, Guilan, Iran.
Niloofar Sojdeh1, Saeid Naderi1*, Alireza Mirzajani2, and Shabnam Shadloo3
1Department of Environmental Sciences, Natural Resources Faculty, University of Guilan, Iran
2Inland Water Aquaculture Research Center, Iranian Fisheries Science Research Institute, Agricultural Research Education and Extension Organization (AREEO), Bandar Anzali, Iran
2Institute of Oceans and Fisheries, University of British Columbia, Canada
*Corresponding Author Email: naderi@guilan.ac.ir
Received 8th April 2024, accepted 22nd June 2024
Abstract: In this study, the diet of the Eurasian otter (Lutra lutra) was investigated in Boujagh National Park for one year. During this investigation, 615 spraints were collected, and the contents of each were identified in the laboratory. To estimate the amount of food items consumption, several statistics, such as percentage of relative frequency of occurrence (RFO%), percentage of frequency of occurrence (PFO%), percentage of relative importance (RI%), and percentage of biomass (Bio%), were calculated. The results showed that fish were the most frequent food item in the species’ diet, and among the fishes, Gobiiformes, Mugiliformes, and Cypriniformes were the most abundant. RFO% were 14%, 12%, and 22% in the warm periods, and 26.94%, 20.23%, and 17.3% in the cold periods, respectively. Such fish species seem valuable because of their size, abundance, and behavioral characteristics. Other taxa, including insects, crustaceans, birds, reptiles, and amphibians were also observed in the otters’ diet. Among them, insects in both warm (RFO=13%) and cold (RFO=6.15%) periods, and reptiles in the warm (RFO=14%) seasons of the year, have had more nutritional importance in Boujagh National Park. The width of the ecological food niche and the diversity of the consumed prey have higher values in the warm seasons. Also, the otter’s food items overlap index indicates a medium value in both warm and cold periods of the year.
Keywords: Ecology, Food Items, Warm and Cold Periods, Spraint Analysis
INTRODUCTION
Carnivores’ foraging habits are important in social structure, habitat use, and reproductive rates, especially if access to food resources is seasonal. In general, if the food resources are abundant, the predator has more options, so it chooses the prey that is easier to hunt and provides more energy. In situations where food resources are plentiful and available, the predator’s diet has less diversity. Conversely, if food resources are limited, the predator often hunts any available prey, which makes the species’ diet more diverse (Stephens and Krebs, 1986; Tinker et al., 2008; Young et al., 2008; Thompson et al., 2014; Garcia-Silva et al., 2020).
The Eurasian Otter (Lutra lutra) is a top predator in aquatic ecosystems. Most research on otters’ diets has been done in freshwater habitats. Only a few studies have investigated the diet and behavior of otters in marine environments. Therefore, more extensive research is needed to determine the importance of marine habitats for this species (Parry et al., 2011).
The food items of Eurasian otters include fish, insects, birds, crustaceans, reptiles, and amphibians, amongst which fish is the main prey. Otters mainly prefer small-sized and slow-moving fish species that are easily caught. The amount of feeding from non-fish alternative prey varies depending on the season and habitats. Most studies show that the degree of flexibility of the Eurasian otter’s diet is directly related to the availability of its prey and habitat. The abundance and variety of prey can affect the width of a predator’s ecological niche. Generally, the predator species that has a significant ecological niche width, has access to more food items (Parry et al., 2011; Kanchanasaka and Duplaix et al., 2011; Gorgadze, 2013; Krawczyk et al., 2016; Bouros et al., 2017; Mirzajani et al., 2021).
Among different otter species, the Eurasian otter is the most widely distributed in the world, but the actual status of this species is unclear. Most of the species’ population was lost due to pollution in the past years, but later, they have recovered, partly through habitat restoration. Currently, this species is classified as Near Threatened in the IUCN Red List and Appendix II of the CITES Organization. As a top predator, otters play an important role in the functioning of aquatic ecosystems (Karami et al., 2006; Novais et al., 2010; Hadipour et al., 2011; Naderi et al., 2017). Their population density, successful reproduction, feeding behavior, and local mortality rate are related to prey availability, which in turn indicates the state of an ecosystem (Reid et al., 2013; Yoxon and Yoxon, 2019) (Fig. 1).
Boujagh National Park is an important habitat for this species in Iran. This park is of great ecological importance due to diverse marine, river, wetland, and estuary ecosystems (the junction of the Sefidroud River with the Caspian Sea). Despite the importance of this species in this region, no study has been done on it so far. In the present study, the diet of the Eurasian otter was studied for one year to analyze the effects of seasonal changes on the animals’ diet.
MATERIALS AND METHODS
Area of Study
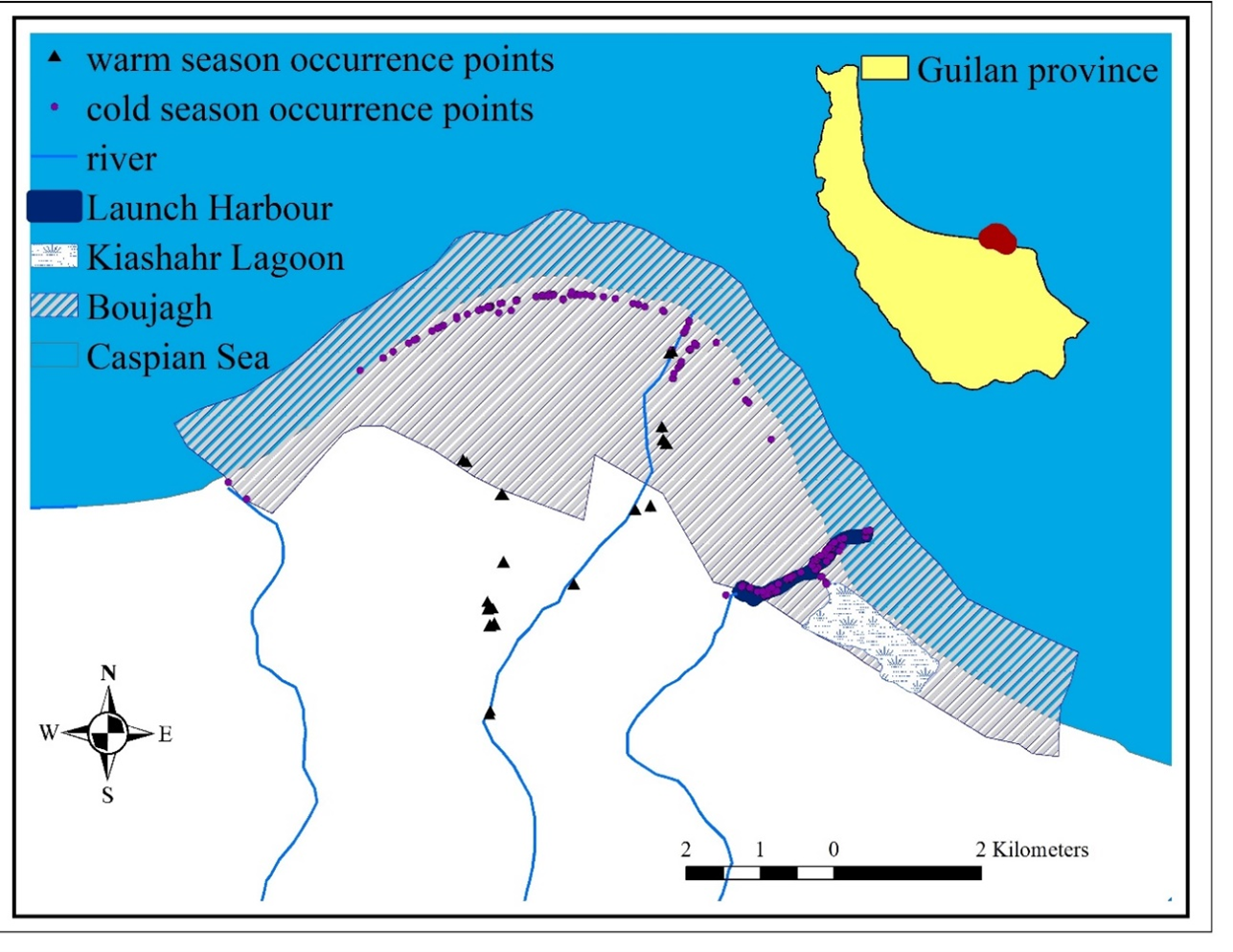
Boujagh National Park is located in the south of the Caspian Sea, Guilan Province, geographical coordinates 49° 51' 40" to 49° 59' 50" E, 37° 25' 00" to 37° 28' 50" N. The national park has a total area of 3278.140 hectares and a circumference of 31.409 km. This area is 23 meters below sea level and is a plain with slopes between 0 and 0.5%. The park’s northern boundary extends to a depth of 6 meters into the Caspian Sea. This park is primarily humid and has two rivers (Sefidroud and Oshmak) and two wetlands (Kiashahr Lagoon and Boujagh wetland). The Kiashahr lagoon, one of the oldest lagoons in Guilan Province, is critical for fish reproduction and bird breeding and wintering areas (Naqinezhad, 2012; Asadi Kapourchal et al., 2014; Saeidi Mehrvarz, 2016) (Fig. 2).
Sampling
The entire area of Boujagh National Park, especially around the wetlands, was investigated, and all observed spraints (615 faeces in total) were collected from July 2018 to June 2019. Collected samples were stored separately in zip-locked plastic envelopes, and the related data of each sample, including date, and geographical coordinates and other descriptive information of the area’s environmental conditions were recorded (Fig. 3).
The spraints were analyzed in a laboratory. First, they were washed through a sieve with a mesh size of 0.5 mm. Then their dry weight was measured by a digital scale (with a sensitivity of 0.01 g). Finally, the samples were entirely washed with water, and their contents were identified in Petri dishes under a stereo microscope and a microscope (Sales-Luís et al., 2007; Hey, 2008; Remonti et al., 2008; Gorgadze, 2013; Mirzaie et al., 2014; Bouros et al., 2017) (Fig. 4).
The prey items were classified into several categories: fish, birds, reptiles, amphibians, insects, and crustaceans. Fish, as the most important prey of the Eurasian otter, were identified to the lowest possible level and split into three categories based on their habitat type (river, marine, and river/marine fishes).
Data Analysis
Diet data were expressed in terms of both frequency and biomass. The relative frequency of occurrence (RFO%) was calculated by dividing the number of each prey item occurrence by the total number of all prey items occurrence. The percentage of frequency of occurrence (PFO%) was calculated by dividing the number of spraints that contained the specific prey by the total number of spraints. Following Wise et al (1981), we also calculated the percentage of Relative Importance (Eq. 1), where Wi is the dry weight of each spraint and Si is the score of each food item, ranging between 1 and 10 (Chuang and Lee, 1996; Bouros et al., 2017).
The percentage of consumed biomass (Bio%), was assessed based on the dry weight of prey remains and the following digestibility coefficients (the ratio of the live prey weight to the remains of that prey in the spraint): 25 for fish, 18 for reptiles and amphibians, 12 for birds, 5 for insects, and 7 for crustaceans (Lockie, 1961; Jedrzejewska et al., 2001; Krawczyk et al., 2016).
The percentage of occurrence of different types of prey was then calculated for each spraint. The data collected from the region were divided into two categories related to warm (July - October 2019, and May - June 2020) and cold periods (November - December 2019, and January - April 2020). Since the data did not follow a normal distribution, the non-parametric Mann-Whitney U Test was used to examine the difference in the percentage of occurrence of each prey item in spraint in two warm and cold periods (Bouros et al. 2017). In addition, in order to compare the difference in percentage of frequency of occurrence and biomass in each group of fish (marine, freshwater, marine-freshwater) and also other prey between warm and cold periods, the non-parametric Mann-Whitney U was used.
The Levins index was used to investigate the width of the food niche of Eurasian otter in two warm and cold periods in Boujagh National Park (Levins, 1968). Using the ratio of food items consumed by the species and Equation 2, the width of the food niche of the species was calculated.
Here, B is the width of Levins’ niche, and Pi is the ratio of each consumed food group. Also, based on Equation 3, the amount of BA, which is the width of the standardized niche, was calculated on a scale of 0 to 1. In this equation, n is the number of food items consumed by the species (Gorgadze, 2013; Bouros et al. 2017).
The Schoener index was calculated to determine the amount of dietary overlap between the year’s warm and cold periods (Schoener, 1974). This index is measured using Equation 4. Here, Cxy is the estimation of overlap value, and P is the ratio of the occurrence of prey I in two warm (x) and cold (y) periods. If the value of this index is zero, it indicates no overlap in the consumed prey species in two periods. If the value of the index is 1, it means complete overlap, while values close to 1 indicate a significant overlap (Garcia-Silva et al. 2020).
The Shannon diversity index determines the aspects of diversity in a community. Using Equation 5, the species diversity of the consumed prey species in the two warm and cold periods was calculated.
Here, Pi is the frequency of occurrence of item i. The higher the value of this index represents the higher species diversity of consumed food items (Clavero et al., 2003).
RESULTS
In general, the diet of Eurasian otter consists of nine orders of fish (Gobiiformes, Mugiliformes, Cypriniformes, Perciformes, Esociformes, Syngnathiformes, Atheriniformes, Clupeiformes, and Salmoniformes), one species of insect from the Coleoptera family, three species of crustaceans (shrimp, Amphipoda, and crab), two bird families (Rallidae and Scolopacidae), a snake and a lizard from reptiles, and frogs from amphibians. We were unable to distinguish species within the fish orders of Gobiiformes and Clupeiformes due to certain limitations, such as sample deterioration and similarities in identification keys for closely related species within these families. Nevertheless, all fish were identified at least to the level of their order (Table 1, Table 2).
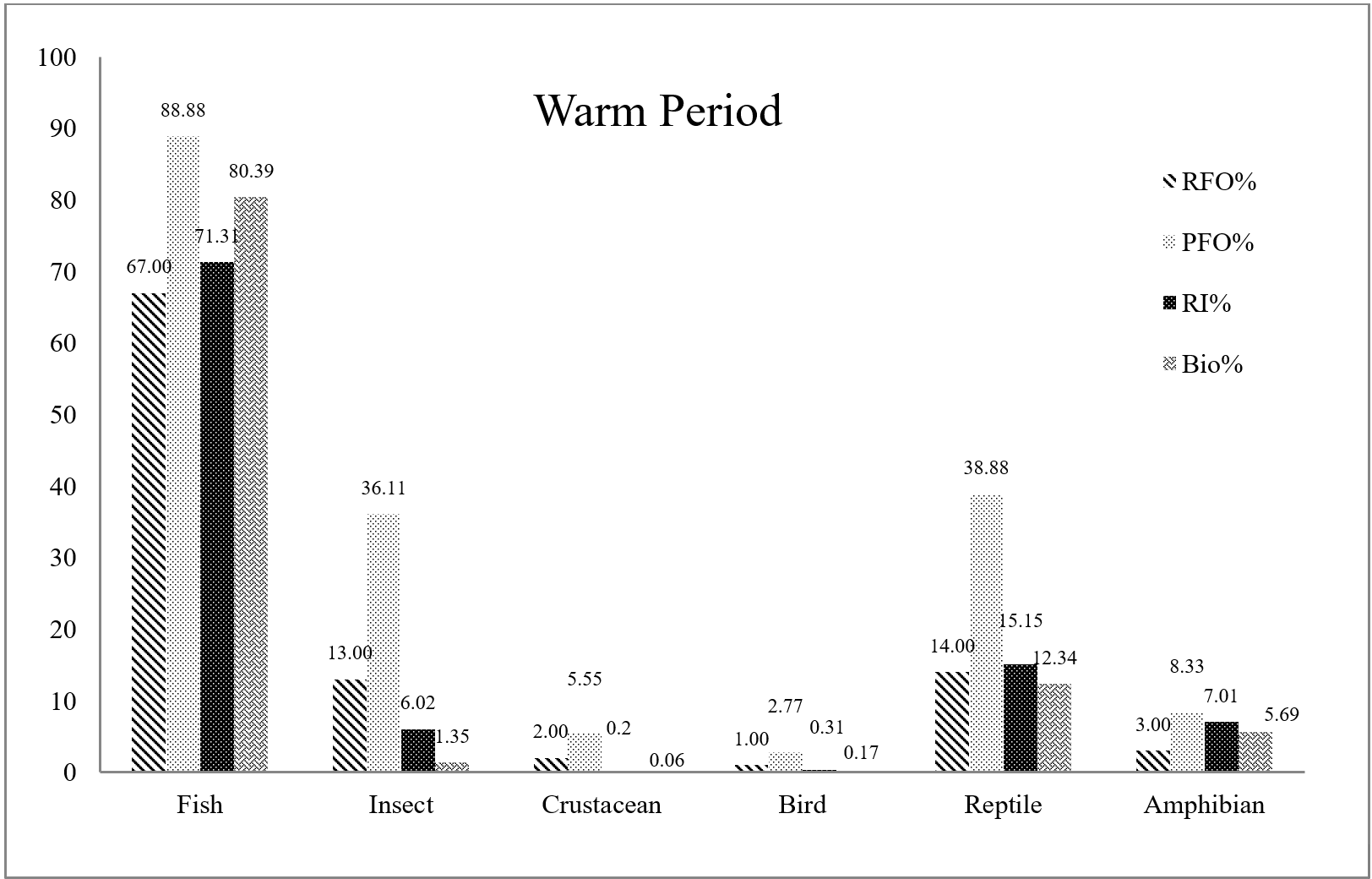
Fish formed the bulk of otter diet in both warm and cold periods of the year. In warm seasons, reptiles and insects ranked second and third. In the cold period, the second rank of importance was related to insects; the rest of the food items were consumed in small quantities . Among the six main food items identified in Eurasian otter spraints, there was no significant difference in the consumption of crustaceans (P=0.488) and birds (P=0.532) between two warm and cold periods of the year. On the other hand, a significant difference was observed in the consumption of fish, insects, reptiles, and amphibians (Table 2).
In both warm and cold periods of the year, the families of Gobiidae, Mugilidae and Cyprinidae were the favorite prey of the otters. Iin the warm period of the year, the Cyprinidae (22%) family had the highest amount of consumption, and two families, Gobiidae (14%), and Mugilidae (12%), were ranked second and third. While among the three families Gobiidae, Mugilidae and Cyprinidae in the cold period of the year, Gobiidae (26.94%), with the highest frequency of occurrence, was the otters’ priority, followed by Mugilidae (20.23%) and Cyprinidae (17.3%) (Table 2).
According to statistical analyses, a significant difference was observed in the most frequently eaten fish species (Gobiiformes, Cypriniformes) between two warm and cold periods (P<0.05). The Mugiliformes and Perciformes orders were also important in the diet of Eurasian otters, but there was no significant difference in their consumption between the two periods (P=0.150; 0.334). Likewise, there was no significant difference in the amount of taking of Esociformes and Atheriniformes (less hunted fish species) between two periods of the year. Syngnathiformes, Clupeiformes, and Salmoniformes were also observed in tiny amounts in Eurasian otter spraints. However, the results show a significant difference in their consumption between the two periods of the year (Table 2).
Among the non-fish prey species, insects (water cockroaches) are a crucial part of the Eurasian otter diet throughout the year. Reptiles (mainly snakes) were only found frequently during the warm period. On the other hand, the amount of bird hunting in the warm period was markedly low, whereas the families of Rallidae and Scolopacidae were identified in small amounts in the cold period. Similarly, shrimps were detected more in the cold period. The rest of the crustaceans, such as Amphipoda and crabs, were caught in small quantities. Frogs were hunted more frequently during the warm period by Eurasian otter (Table 2) (Fig. 5).
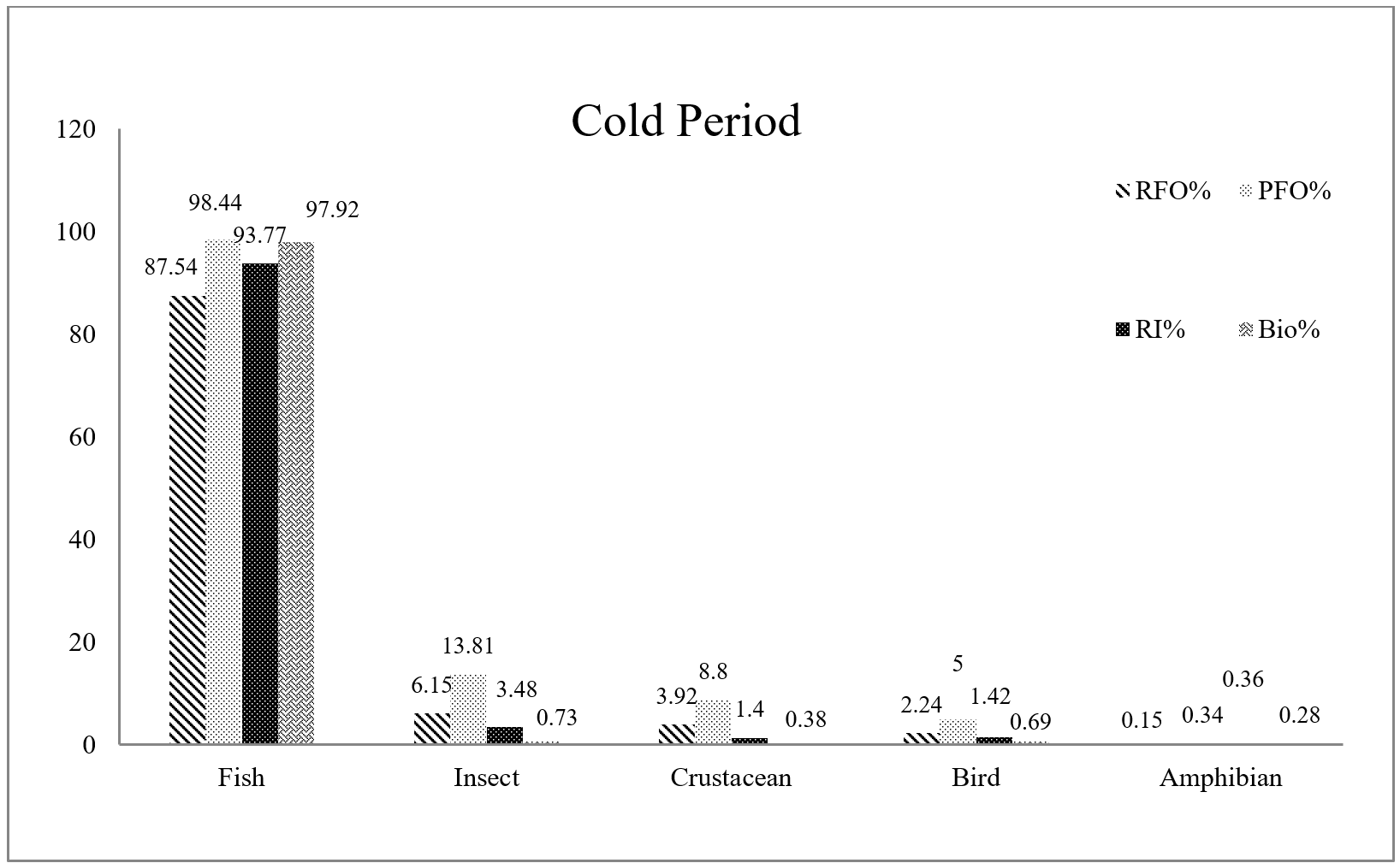
According to the results, Eurasian otters hunt differently in marine and riverine ecosystems. In the warm period, the highest abundance of prey remains are freshwater fish (28.95%). After that, marine fish (26.31%) and non-fish prey (26.31%) were the most abundant. Fish species that are common in both rivers and the Caspian Sea included 18.42% of the otters’ diet. However, the results are slightly different in terms of the percentage of biomass consumed; marine fishes (44.22%) have the first rank, and freshwater fishes (29.43%), non-fish prey (19.61%), and fish inhabiting both the rivers and the Caspian Sea (6.74%). Also, in the cold period, the highest frequency of prey occurrence in Eurasian otter diet was for the fish order Gobiiformes, and Gasterosteidae family (Perciformes), which are common species in the Caspian Sea and rivers (35.03%). Marine and freshwater fish species (28.26%, and 24.12% respectively), and non-fish species (12.58%) followed in consumption rank (Table 3).
According to the statistical analysis based on the frequency of occurrence of each prey in the spraints, there was a significant difference between the warm and cold period in the consumption of common marine-freshwater fish species and non-fish species (P-value=0). There was no significant difference between the warm and cold period in the frequency of consumption of marine and freshwater prey. The results obtained from the analysis of biomass are also similar to the frequency of occurrence. There is only a difference in relation to freshwater fish, where a significant difference was seen between the warm and cold periods of the year (P=0.007) (Table 3).
The width of the food niche (B) of Eurasian otter in Boujagh National Park is larger in the warm period of the year. The standard food niche (BA) and the overall Shannon diversity index are also higher in the warm period. The estimated value of the Schoener index (0.63) indicates the medium overlap between the Eurasian otter’s diet in warm and cold periods in Boujagh National Park (Table 4).
| Table 4: Fish prey identified in the Eurasian otter diet in Boujagh National Park | |||
| Warm Period | Cold Period | ||
| N | 36 | 579 | |
| B | 7.996 | 5.978 | |
| BA | 0.349 | 0.248 | |
| Shannon diversity index | 2.31 | 2.02 | |
| Schoener index | 0.63 | ||
DISCUSSION
Using indirect methods to estimate the diet of a predator species requires caution. Most of the information obtained from these studies can create a general and approximate picture of the nutritional behavior of the target species. However, the accuracy of this analysis is affected by the method used to estimate the diet and many related complicated factors (Clavero et al., 2004; Remonti et al., 2008; Lanszki et al., 2015; Bouros et al., 2017).
During the year, fish always form the most significant amount of the species’ diet, and are always their preferred food, but there is still a difference in the amount of their consumption in the two periods. Our study shows that the amount of fish consumption decreases in the warm season. This reduction is because chasing and hunting fish in warmer water needs more energy, because with the increase of water temperature, fish species’ metabolism rates and swimming speed increases. On the other hand, during the warm season, more alternative prey are available to the otters (Clavero et al., 2003; Brzeziński et al., 2006; Bauer-Haáz et al., 2014).
According to a study by Mason and Macdonald (1986), feeding on fish depends on their size and availability. Smaller fish (less than 200 mm) are more dominant in the otters’ diet. During the two periods, the consumption of Gobiiformes was more than that of other fish orders. The small size of Gobiiformes species (often between 50 and 100 mm), their abundance in the aquatic ecosystems of Boujagh National Park, and their relatively slower swimming speed can influence their hunting rate by Eurasian otters.
Two orders of Cypriniformes and Mugiliformes were most preferred by Eurasian otters. Among the Cypriniformes fishes, Prussian carp (Carassius gibelio) was the most abundant in spraint. This species is relatively small in size. Because young and immature otters cannot hunt larger prey, they mainly target smaller fish. Despite the higher consumption of small-sized fish by otter, they do sometimes feed on the larger fish (greater than 1000 grams) (McMahon and McCafferty, 2006; Gorgadze, 2013; Lanszki et al., 2015).
Seasonal variation in reptile consumption was consistent with their availability, as during the cold period reptiles are less active, and often hibernating. However, it is different for amphibians. Amphibians were hunted in small amounts in cold seasons despite hibernation. This can happen for several reasons. Firstly, during cold seasons, some temporary warm periods can make frogs come out of their hibernation places to small ponds in a dozy state, making them easy prey for otters. Moreover, otters can take advantage of the opportunity when amphibians hibernate under stones and sticks, as they can turn them over with their snouts and feed on the sleeping amphibians. Furthermore, the end of the cold period (March and April) coincides with the beginning of the frogs’ mating season, during which they make elaborate noises to attract mates, which can also attract otters as predators. Finally, in warm seasons, amphibians inhabit spawning waters, ponds, and other bodies of water for breeding, making them accessible prey for otters (Weber, 1990; Sulkava, 1996; Britton et al., 2006; Brzeziński et al., 2006; Cousins et al., 2011; Krawczyk et al., 2016; Sittenthaler et al., 2019; Andeska et al., 2021).
Birds formed only a tiny fraction of their diet. Among the bird species, coots (Fulica atra) were found in the highest proportion. It may be due to their nesting behavior as they build their nests on the ground and often near the shore and reeds. They also usually loaf on the water’s edge and the emergent plants (Chanin, 1981; Irwin et al., 1997; Hey, 2008).
In contrast to previous research findings where mammals were noted as part of Eurasian otter diet (Remonti et al., 2008; Mirzaie et al., 2014; Lanszki et al., 2015), in the present investigation within Boujagh National Park, there was no evidence or traces of mammal consumption.
Conroy and Jenkins (1986) and Beja (1991) stated that preying on fish in marine environments requires more energy than freshwater, so otters prefer to feed in freshwater. However, in the present study, marine and marine-riverine fish species were the most significant part of the diet, indicating the dependence of otters on both freshwater and marine environments. It seems that changes in the abundance of fish populations in different seasons, secure access to food resources, and probably habitat alterations affect on the trends of this process (Clavero et al., 2004; Parry et al., 2011).
Based on the findings, the food niche breadth and Shannon diversity index are significantly greater in the warm period than to the cold period. This is attributed to a relative decrease in fish abundance during the warm season, which forces otters to expand their food niche in order to provide sufficient energy and consume a wider range of items. Conversely, the warm period sees an increase in the availability of alternative prey such as reptiles and other species, further contributing to the expanded dietary options for otters. These dietary changes lead to an increase in Shannon’s diversity index during the warm period. On the contrary, during the cold period, due to the high levels of primary food sources, Eurasian otters often hunt for fish, which reduces dietary diversity and nutritional items. Because fish provide more energy than other food items and are highly available in cold seasons, they comprise much of the species’ diet. This makes the food niche width and Shannon index less in this period. Similar results have been obtained in previous studies (Brzeziński et al., 1993; Baltrūnaitė, 2006; Georgiev, 2006; Gorgadze, 2013).
As previously explained, the diversity of species eaten by otters in warm seasons is greater than in cold seasons. This difference in the variety of the eaten prey species has therefore led to the medium overlap of dietary items in two periods (Schoener = 0.6). This result is consistent with the data of some other studies (Brzeziński et al., 1993; Baltrūnaitė, 2006)..
Despite Eurasian otters being specialized for feeding on fish, the opportunistic behavior of this species has been proved in many dietary studies: when fish density in an area decreases, otters turn to feeding on other prey species (Sulkava, 1996; Jedrzejewska et al., 2001; Brzeziński et al., 2006). Its feeding habits therefore vary depending on the time and environmental conditions. Providing an environment with the least stress and the highest food resources can effectively conserve this valuable species’ population.CONCLUSION
In conclusion, since this species prefers to feed on fish, it is essential to investigate the abundance and behavior of fish in the waters of Boujagh National Park, considering the species’ foraging behavior, especially during the breeding season. This can be pivotal for planning of applied conservation program for this critical species.
Acknowledgments - We are very grateful to the dear late Amir Ebrahimi, for his cooperation and guides in most of the field surveys. Unfortunately, some time after the completion of this study, we lost him due to an accident in nature. He was a great and committed person and a pure lover of nature, and he is known as “Boujagh observatory” thanks to his countless environmental services in various fields in Kiashahr region and Boujagh National Park. May the soul of that great man rest in peace.
REFERENCES
Andeska, F., Novarino, W., Nurdin, J. and Aadrean (2021). Relationship between temporal environment factors and diet composition of small-clawed otter (Aonyx cinereus) in heterogeneous paddy fields landscape in Sumatra, Indonesia. IUCN Otter Specialist Group Bulletin, 38 (2): 106-116. https://iucnosgbull.org/Volume38/Andeska_et_al_2021.html
Asadi Kapourchal, S., Dehdar Dargahi, M., Karimzadegan, H. (2014). Zoning coastal marine Boujagh National Park and sustainable management by geographic information system. Indian Journal of Science and Technology, 7 (12): 1933-1938. http://dx.doi.org/10.17485/ijst/2014/v7i12.13
Baltrūnaitė, L. (2006). Seasonal diet of the otter (Lutra lutra L.) in natural river ecosystems of south-eastern Lithuania. Acta Zoologica Lituanica, 16 (2), 107-114. https://doi.org/10.1080/13921657.2006.10512717
Bauer-Haáz, É.A., Ferincz, Á., Szegvári, Z., Széles, G.L., Lanszki, J. (2014). Fish preference of the eurasian otter (Lutra lutra) on an abandoned fish pond and the role of fish sampling methods. Fundamental and Applied Limnology, 184 (2): 161-168. http://dx.doi.org/10.1127/1863-9135/2014/0616
Beja, P.R. (1991). Diet of otters (Lutra lutra) in closely associated freshwater, brackish and marine habitats in south-west Portugal. Journal of Zoology, 225 (1): 141-152. https://doi.org/10.1111/j.1469-7998.1991.tb03807.x
Bouros, G., Murariu, D. (2017). Comparative diet analysis of the eurasian otter (Lutra lutra) in different habitats: Putna-Vrancea Natural Park and Lower Siret Valley, south-eastern Romania. North-Western Journal of Zoology, 13 (2): 311-319. https://biozoojournals.ro/nwjz/content/v13n2/nwjz_e161704_Bouros.pdf
Britton, R., Pegg, J., Shepherd, J.S., Toms, S. (2006). Revealing the prey items of the otter Lutra lutra in southwest England using stomach contents analysis. Folia Zoologica, 55 (2): 167-174. https://www.ivb.cz/wp-content/uploads/55_167-174.pdf
Brzeziński, M., Jedrzejewski, W., Jedrzejewska, B. (1993). Diet of otters (Lutra lutra) inhabiting small rivers in the Bialowieza national park, eastern Poland. Journal of Zoology. 230: 495-501. https://doi.org/10.1111/j.1469-7998.1993.tb02701.x
Brzeziński, M., Romanowski, J., Kopczyński, Ł., Kurowicka, E. (2006). Habitat and seasonal variations in diet of otters, Lutra lutra in eastern Poland. Folia Zoologica, 55 (4): 337-348. https://www.peeref.com/works/79727241
Chanin, P. (1981). The diet of otter and it relations with the feral mink in two areas of southwest England. Acta Theriologica, 26 (5): 83-95. https://rcin.org.pl/ibs/publication/26996
Chuang, S and Lee, L. (1996). Food habits of three carnivore species (Viverricula indica, Herpestes urva, and Melogale moschata) in Fushan Forest, northern Taiwan. Journal of Zoology, 243 (1): 71-79. https://doi.org/10.1111/j.1469-7998.1997.tb05757.x
Clavero, M., Prenda, J., Delibes, M. (2003). Trophic diversity of the otter (Lutra lutra) in temperate and mediterranean freshwater habitats. Journal of Biogeography, 30 (5): 761-769. https://doi.org/10.1046/j.1365-2699.2003.00865.x
Clavero, M., Prenda, J., Delibes, M. (2004). Influence of spatial heterogeneity on coastal otter (Lutra lutra) prey consumption. Annales Zoologici Fennici, 41 (4): 551-561. https://www.sekj.org/PDF/anzf41/anzf41-551.pdf
Conroy, J.W.H., Jenkins, D. (1986). Ecology of otters in northern Scotland. VI. Diving times and hunting success of otters (Lutra lutra) at Dinnet Lochs, Aberdeenshire and in Yell Sound, Shetland. Journal of Zoology, 209 (3): 341-346. https://doi.org/10.1111/j.1469-7998.1986.tb03597.x
Cousins, L., Tansley, D. and Hepburn, L. (2011). Investigation into the dietary habits of the Eurasian otter (Lutra lutra) in the County of Essex. IUCN Otter Specialist Group Bulletin, 28 (2): 76-83. https://www.iucnosgbull.org/Volume28/Cousins_et_al_2011.html
Garcia-Silva, O., Gallo-Reynoso, J.P., Bucio-Pacheco, M., Medrano-López, J.M., Meza-Inostroza, P.M., Grave-Partida, R.A. (2020). Neotropical otter diet variation between a lentic and a lotic systems. Therya, 12 (1): 95-103. http://dx.doi.org/10.12933/therya-21-781
Georgiev, D.G. (2006). Diet of the otter Lutra lutra in different habitats of south-eastern Bulgaria. IUCN Otter Specialist Group Bulletin, 23 (1): 5-11. https://www.iucnosgbull.org/Volume23/Georgiev_2006.html
Gorgadze, G. (2013). Seasonal diet of the otter (Lutra lutra) on the Alazani river (Georgia). Hystrix, 24: 157-160. https://doi.org/10.4404/hystrix-24.2-4685
Hadipour, E., Karami, M., Abdoli, A., Riazi, B., Goljani, R. (2011). A study on Eurasian otter (Lutra lutra) in Amirkelayeh Wildlife Refuge and International Wetland in Guilan province, northern Iran. IUCN Otter Specialist Group Bulletin, 28 (2): 84-98. https://www.iucnosgbull.org/Volume28/Hadipour_et_al_2011.html
Hey, D.C. (2008). The importance of birds in the diet of otter Lutra lutra on Shapwick Heath. Bioscience Horizons, 1: 143-147. https://doi.org/10.1093/biohorizons/hzn018
Irwin, S., O’Halloran, J. (1997). The wintering behavior of coot Fulica atra at Cork Lough, south west Ireland. Journal of Environmental Biology, 97 (2): 157-162. https://www.ucc.ie/en/media/research/planforbio/pdfs/publications/03.IrwinOHalloran1997BandE.pdf
Jedrzejewska, B., Sidorovich, V.E., Pikulik, M.M., Jedrzejewski, W. (2001). Feeding habits of the otter and american mink in Białowieza Primeval Forest (Poland) compared to other Eurasian populations. Ecography, 24 (2): 165-180. https://www.jstor.org/stable/3683692
Kanchanasaka, B. and Duplaix, N. (2011). Food habits of the hairy-nosed otter (Lutra sumatrana) and the small-clawed otter (Aonyx cinereus) in Pru Toa Daeng Peat Swamp forest, southern Thailand. IUCN Otter Specialist Group Bulletin, 28 (A): 139-161. https://iucnosgbull.org/Volume28A/Kanchanasaka_Duplaix_2011.html
Karami, M., Mirzaei, R., Hamzehpour, M. (2006). Status of Eurasian otter (Lutra lutra) in Iran. IUCN Otter Specialist Group Bulletin, 23 (1): 27-33. https://iucnosgbull.org/Volume23/Karami_et_al_2006.html
Krawczyk, A.J., Bogdziewicz, M., Majkowska, K., Glazaczow, A. (2016). Diet composition of the Eurasian otter Lutra lutra in different freshwater habitats of temperate Europe: A review and meta-analysis. Mammal Review, 46 (2): 106-113. https://doi.org/10.1111/mam.12054
Lanszki, J., Bauer-Haáz, É.A., Széles, G.L., Heltai, M. (2015). Diet and feeding habits of the Eurasian otter (Lutra lutra): Experiences from post mortem analysis. Mammal Study, 40 (1): 1-11. https://doi.org/10.3106/041.040.0102
Levins, R. (1968). Evolution in changing environments: Some theoretical explorations. Princeton University Press, Princeton. https://dokumen.pub/evolution-in-changing-environments-some-theoretical-explorations-mpb-2-9780691209418.html
Lockie, J.D. (1961). The food of the pine marten Martes martes in west Ross Shire, Scotland. Proceedings of the Zoological Society of London, 136 (2): 187-195. https://doi.org/10.1111/j.1469-7998.1961.tb06171.x
Mason, C.F and Macdonald, S.M. (1986). Otters ecology and conservation. Cambridge University Press, Cambridge.
McMahon, J and McCafferty, D.J. (2006). Distribution and diet of otters (Lutra lutra) in marine areas of Loch Lomond and The Trossachs National Park, Scotland, UK. Lutra, 49 (1): 26-36. https://www.zoogdiervereniging.nl/~zoogdier/sites/default/files/publications/Lutra%2049%281%29_McMahon%20%26%20McCafferty_2006.pdf
Mirzaie, R., Danehkar, A., Abdoli, A. (2014). The diet of Eurasian otters in the Jajrood river system, Iran. Mammal Study, 39 (1): 33-41. http://dx.doi.org/10.3106/041.039.0106
Mirzajani, A., Naderi, S., Ganeh, A., Hadipour, E., Salahi, M., Javidpour, J. (2021). Trophic flexibility of Eurasian otter (Lutra lutra) in Anzali Wetland, Iran, assessed by fecal and stable isotope analysis. Aquatic Ecology, 55 (2): 401-415. http://dx.doi.org/10.1007/s10452-021-09832-x
Naderi, S., Mirzajani, A., Hadipour, E. (2017). Distribution of and threats to the Eurasian otter (Lutra lutra) in the Anzali Wetland, Iran. IUCN Otter Specialist Group Bulletin, 34 (2): 84-94. https://www.iucnosgbull.org/Volume34/Naderi_et_al_2017.html
Naqinezhad, A. (2012). A physiognomic-ecological vegetation mapping of Boujagh National Park, the first marine-land national park in Iran. Advanced Biomedical Research, 3 (1): 37-42. https://soeagra.com/abr/abr_march2012/10.pdf
Novais, A., Sedlmayr, A., Moreira-Santos, M., Goncalves, F., Ribeiro, R. (2010). Diet of the otter Lutra lutra in an almost pristine Portuguese river: Seasonality and analysis of fish prey through scale and vertebrae keys and length relationships. Journal of Mammalian Evolution, 74: 71-81. https://doi.org/10.1515/mamm.2010.010
Parry, G.S., Burton, S., Cox, B., Dan, W., Forman, D.W. (2011). Diet of coastal foraging Eurasian otters (Lutra lutra) in Pembrokeshire south-west Wales. European Journal of Wildlife Research, 57 (3): 485-494. http://dx.doi.org/10.1007/s10344-010-0457-y
Reid, N., Thompson, D., Hayden, B., Marnell, F., Montgomery, I. (2013). Review and quantitative meta-analysis of diet suggests the Eurasian otter (Lutra lutra) is likely to be a poor bioindicator. Elsevier, 26: 5-13. http://dx.doi.org/10.1016/j.ecolind.2012.10.017
Reihanian, A., Hin, T.W., Kahrom, E., Binti Mahmood, N.Z. (2015a). A framework for implementing sustainable tourism in national parks of Iran: development and use of sustainable tourism indicators in Boujagh National Park, Iran. Caspian Journal of Environmental Sciences (CJES), 13: 41-52. https://cjes.guilan.ac.ir/article_202.html
Reihanian, A., Hin, T.W., Kahrom, E., Binti Mahmood, N.Z., Bagherpour Porshokouh, A. (2015b). An examination of the effects of push and pull factors on Iranian national parks: Boujagh National Park, Iran. Caspian Journal of Environmental Sciences (CJES), 13 (3): 197-206. https://cjes.guilan.ac.ir/article_1371.html
Remonti, L., Prigioni, C., Balestrieri, A., Sgrosso, S., Priore, G. (2008). Trophic flexibility of the otter (Lutra lutra) in southern Italy. Mammalian Biology, 73 (4): 293-302. http://dx.doi.org/10.1016/j.mambio.2007.04.004
Saeidi Mehrvarz, S., Naqinezhad, A., Ravanbakhsh, M., Maghsoudi, M. (2016). The survey of macrophytes diversity in wetland zone of Boujagh National Park, Guilan, Iran. Asian Journal of Conservation Biology, 5 (2): 75-80. https://www.ajcb.in/journals/full_papers_dec_2016/AJCB-Vol5-No2-%20Mehrvarz%20et%20al.pdf
Sales-Luís, T., Pedroso, N.M., Santos-Reis, M. (2007). Prey availability and diet of the Eurasian otter (Lutra lutra) on a large reservoir and associated tributaries. Canadian Journal of Zoology, 85 (11): 1125-1135. https://doi.org/10.1139/Z07-087
Schoener, T.W. (1974). Resource partitioning in ecological communities. Science, 185 (4145): 27-39. https://doi.org/10.1126/science.185.4145.27
Sittenthaler, M., Koskoff, L., Pinter, K., Nopp-Mayr, U., Parz-Gollner, R., Hackländer, K. (2019). Fish size selection and diet composition of Eurasian otters (Lutra lutra) in salmonid streams: Picky gourmets rather than opportunists. Knowledge and Management of Aquatic Ecosystems, 420: 29. http://dx.doi.org/10.1051/kmae/2019020
Stephens, D.W and Krebs, J.R. (1986). Foraging theory. Princeton University Press. New Jersey, USA. https://doi.org/10.2307/1381654
Sulkava, R. (1996). Diet of otters Lutra lutra in central Finland. Acta Theriologica, 41: 395-408. http://dx.doi.org/10.4098/AT.arch.96-38
Thompson, L, and Stelle, L.L. (2014). Prey preference of the north American river otter (Lontra canadensis) evaluated based on optimal foraging theory. IUCN Otter Specialist Group Bulletin, 31 (1): 15-29. https://www.iucnosgbull.org/Volume31/Thompson_Stelle_2014.html
Tinker, M.T., Bentall, G., Estes, J.A. (2008). Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proceedings of the National Academy of Sciences (PNAS), 105 (2): 560-565. https://doi.org/10.1073/pnas.0709263105
Weber, J.M. (1990). Seasonal exploitation of amphibians by otters Lutra lutra in north-east Scotland. Journal of Zoology, 220 (4): 641-651. http://dx.doi.org/10.1111/j.1469-7998.1990.tb04740.x
Wise, M.H., Linn, I.J., Kennedy, C.R. (1981). A comparison of the feeding biology of mink Mustela vison and otter Lutra lutra. Journal of Zoology, 195 (2): 181-213. https://doi.org/10.1111/j.1469-7998.1981.tb03458.x
Young, J.K., Glasscock, S.N., Shivik, J.A. (2008). Does spatial structure persist despite resource and population changes? Effects of experimental manipulation on coyotes. Journal of Mammalogy, 89 (5): 1094-1104. http://dx.doi.org/10.1644/07-MAMM-A-198.1
Yoxon, P and Yoxon, B. (2019). Eurasian otter (Lutra lutra): A review of the current world status. Journal International Otter Survival Fund, 53-73. https://www.researchgate.net/publication/333699604_EURASIAN_OTTER_Lutra_lutra_A_REVIEW_OF_THE_CURRENT_WORLD_STATUS
Résumé: Régime Alimentaire de la Loutre Eurasienne (Lutra lutra) dans le Parc National de Boujagh, Guilan, Iran
Dans cette étude, le régime alimentaire de la loutre eurasienne (Lutra lutra) a été étudié dans le Parc National de Boujagh pendant un an. Au cours de cette investigation, 615 excréments ont été collectées et le contenu de chacune d’entre elles a été identifié en laboratoire. Pour estimer la quantité de produits alimentaires consommés, plusieurs statistiques telles que le pourcentage de fréquence relative d’occurrence (FRO), le pourcentage de fréquence d’occurrence (PFO), le pourcentage d’importance relative (IR%) et le pourcentage de biomasse (Bio%) ont été calculés. Les résultats ont montré que les poissons étaient l’aliment le plus fréquent dans le régime alimentaire de l’espèce et que parmi les poissons, les Gobiiformes, les Mugiliformes et les Cypriniformes étaient les plus abondants et que le pourcentage de RFO était de 14 %, 12 % et 22 % pendant les périodes chaudes, et de 26,94 %, 20,23 % et 17,3 % pendant les périodes froides, respectivement. Ces espèces de poissons semblent précieuses en raison de leur taille, de leur abondance et de leurs caractéristiques comportementales. D’autres taxons, notamment des insectes, des crustacés, des oiseaux, des reptiles et des amphibiens, ont également été observés dans le régime alimentaire des loutres. Parmi eux, les insectes pendant les périodes chaudes (RFO=13%) et froides (RFO=6.15%), ainsi que les reptiles pendant les saisons chaudes (RFO=14%) de l’année, ont eu une plus grande importance nutritionnelle dans le parc national de Boujagh. La largeur de la niche alimentaire écologique et la diversité des proies consommées ont des valeurs plus élevées pendant les saisons chaudes. De plus, l’indice de chevauchement des aliments de la loutre indique une valeur moyenne aussi bien dans les périodes chaudes que froides de l’année.
Revenez au dessus
Resumen: Dieta de la Nutria Eurasiática (Lutra lutra) en el Parque Nacional Boujagh, Guilan, Irán
En éste estudio, se investigó la dieta de la Nutria Eurasiática (Lutra lutra) en el Parque Nacional Boujagh, durante un año. Durante ésta investigación fueron colectadas 615 fecas, y se identificó el contenido de cada una en el laboratorio. Para estimar el monto del consumo de los items alimentarios, se calcularon diversos estadísticos, como el porcentaje de frecuencia relativa de ocurrencia (RFO%), porcentaje de frecuencia de ocurrencia (PFO%), porcentaje de importancia relativa (RI%), y porcentaje de biomasa (Bio%). Los resultados mostraron que los peces son el item alimentario más frecuente en la dieta de la especie, y entre los peces, los más abundantes fueron los Gobiiformes, Mugiliformes, y Cypriniformes, con RFO% de 14%, 12%, y 22% en los períodos cálidos, y 26.94%, 20.23%, y 17.3% en los períodos fríos, respectivamente. Estas especies de peces parecen ser valiosas en la dieta a causa de su tamaño, abundancia, y características de comportamiento. Otros taxones, incluyendo insectos, crustáceos, aves, reptiles, y anfibios también fueron observados en la dieta de las nutrias. Entre ellos, los insectos tanto en períodos cálidos (RFO=13%) como fríos (RFO=6.15%), y los reptiles en las estaciones cálidas del año (RFO=14%), tuvieron mayor importancia nutricional en el Parque Nacional Boujagh. La amplitud del nicho ecológico alimentario y la diversidad de las presas consumidas mostraron valor más altos en las estaciones cálidas. También, el índice de superposición de items alimentarios indica un valor medio tanto en períodos cálidos como fríos.
Vuelva a la tapa
چکیده
در مطالعه حاضر، رژیم غذایی گونه شنگ اوراسیایی (Lutra lutra)، در پارک ملی بوجاق برای یک سال، بررسی شد. در طی این بررسی، 615 مدفوع جمع آوری شدند و محتویات هر یک از آن ها، در آزمایشگاه، شناسایی شدند. برای تخمین مقدار مصرف آیتم های غذایی، چندین آماره مختلف مانند درصد فراوانی نسبی وقوع (RFO%)، درصد فراوانی وقوع (PFO%)، درصد ارجحیت نسبی (RI%)، و درصد بیوماس (Bio%)، محاسبه شدند. نتایج، حاکی از آن بود که ماهی فراوان ترین آیتم رژیم غذایی گونه بوده، و در میان راسته های ماهی، گاوماهیان، کفال ماهیان و کپورماهیان، فراوان ترین بودند و مقدار RFO در دوره های گرم به ترتیب %14، %12 و %22 و در دوره های سرد %26.94، %20.23 و %17.3 درصد بود. چنین ماهیانی، به نظر می رسد بخاطر اندازه، فراوانی و خصوصیات رفتاری، برای شنگ ها ارزشمند هستند. سایر موجودات، شامل حشرات، سخت پوستان، پرندگان، خزندگان، و دوزیستان نیز در رژیم غذایی شنگ مشاهده شدند. در میان آن ها، حشرات در هر دو دوره گرم(RFO=13%)و سرد(RFO=6.15%)، و خزندگان، در فصول گرم(RFO=14%)سال، بیشترین ارزش تغذیه ای را در پارک ملی بوجاق، برای شنگ داشتند. پهنای آشیان اکولوژیک غذایی و تنوع طعمه های مصرف شده در فصول گرم سال، دارای مقادیر بالاتری بودند. همچنین، شاخص همپوشانی آیتم های غذایی شنگ، مقدار متوسطی را در هر دو دوره گرم و سرد سال نشان داد.
بازگشت به ابتدا