IUCN/SSC Otter Specialist Group Bulletin

©IUCN/SCC Otter Specialist Group
Citation: White, J., Chueva, C., and Latamsathit, T.(2025). Otter Density Correlates with Elevation in the Northern Annamite Mountains of Laos . IUCN Otter Spec. Group Bull. 42 (3): 133 - 150
Otter Density Correlates with Elevation in the Northern Annamite Mountains of Laos
Jay White*, Chayyang Chueva, and Thongla Latamsathit
Wildlife Conservation Society, Lao PDR P.O. Box 6712 House No 390, Unit 34, Naxay Village, Xaysettha District, Vientiane, 01000, Lao PDR
*Corresponding Author Email: jaywhite@wcs.org
Received 18th May 2025, accepted 1st July
Abstract: Three species of otter have been identified in the People’s Democratic Republic of Lao (Laos): the smooth-coated otter (Lutrogale perspicillata), the Eurasian otter (Lutra lutra), and the small-clawed otter (Aonyx cinereus). All three species have experienced significant population declines and range constrictions globally and in Laos and require targeted conservation action to remain viable. We examined species composition and distribution in a protected area in the northern Annamite Mountains of Laos. We used camera trap detections and population density (by proxy of spraint density) to compare the significance of several natural and anthropogenic variables in predicting density and distribution. We hypothesized that we would find evidence of Eurasian and small-clawed otter at the study site and that they would show signs of niche segregation based on stream size and elevation– with small-clawed otter showing a preference for small high elevation streams and Eurasian otter, the opposite. We identified two species of otter: small-clawed and Eurasian otter but did not observe evidence of niche segregation between them. We did find a significant negative correlation of combined otter density (both species) with elevation, both species showing a preference for streams at lower elevations in the site. Additionally, we found no evidence of spatial avoidance of human activity. This suggests that lowland streams, including streams outside protected areas in Laos, are critical for otter conservation and deserve protection efforts, while high elevation streams may be more marginal as otter habitat.
Keywords: small-clawed otter, Eurasian otter, spraint density, general linear models, protected area, camera trapping
INTRODUCTION
Molecular approaches have revolutionized the fields of genetics, ecology, and evolutionary biology, and are routinely applied in surveys, monitoring programs and status assessments of threatened organisms (Schwartz et al., 2007). However, the application of such methods to wildlife species that are rare, elusive, and/or poorly studied is often hindered by the paucity of appropriate molecular markers for efficient genetic analysis. Therefore, developing molecular tools that are specifically designed for poorly known taxa is a useful avenue to enable genetic, ecological, and conservation-oriented studies targeting these species.
The small-clawed otter is the world’s smallest otter and is adapted to shallow streams and water bodies. As its name implies, its claws are much smaller than those of most other otter species, an adaptation which improves its ability to find and catch aquatic invertebrates, particularly crabs (Hussain et al., 2011; Duplaix and Savage, 2018). The smooth-coated otter is the largest of these three species, recognizable by its size and short-haired smooth pelt; its claws are longer and its feet more webbed than the small-clawed otter which makes it better adapted for fishing in larger lowland water bodies (Anoop and Hussain, 2004). The Eurasian otter, like the smooth-coated otter, is adapted to fishing but also consumes a wide array of other prey including amphibians, and tends to consume smaller fish than the smooth-coated otter when sharing habitat (Kruuk et al., 1994).
All three otter species have experienced significant population declines and range constrictions in the last century due to habitat loss, depletion of prey from overfishing, persecution due to perceived competition over fish stocks, hunting for the wildlife trade, and capture for the pet trade (Duplaix and Savage, 2018). All three species are on the IUCN Red List - small-clawed and smooth-coated otters are listed as Vulnerable (Khoo et al., 2020; Wright et al., 2021) and Eurasian otter as Near Threatened (Loy, A. et al., 2020); all three are listed in Appendix I of the Convention on International Trade of Endangered Species (CITES, 2024); and all three species are in Category I, the highest protection category, in the Lao Wildlife Law (Ministry of Agriculture and Forestry, 2023). Despite the protection, otters are still traded in Laos (Schweikhard et al., 2019) and more broadly in Asia, primarily as pets or for their pelts; the species most heavily traded as a pet being the small-clawed otter while trafficked pelts are mainly those of Eurasian and smooth-coated otters (Gomez et al., 2016; Gomez and Bouhuys, 2018).
We examined the species composition and distribution of otters in a protected area in the northern Annamite Mountains of Laos. Our study site is currently under a management plan involving ongoing monitoring of target species and clades (otters being one) to assess conservation impact. This survey is the first of several to monitor otter population dynamics and thus serves as an initial assessment and baseline of otters in the site. Additionally, we examined how distribution of the site’s otter species relates to natural and anthropogenic variables in order to assist otter conservation efforts there and in South East Asia more broadly.
Based on survey results in Nakai Nam Thuen National Park (about 100 km southeast of our site) from Coudrat et al. (2022), we hypothesized that we would find small-clawed and Eurasian otters and not smooth-coated otter. We also hypothesized, based on previous reports (Kruuk et al., 1994; Prenda and Granado-Lorencio, 1996; Hussain and Choudhury, 1997; Perinchery et al., 2011), that small-clawed otter distribution would reflect a preference for smaller streams at higher elevation while Eurasian otter distribution would reflect a preference for larger lower elevation streams. To test these hypotheses, we used camera traps to examine the distribution of the species, and spraint counts to compare relative otter densities across the protected area. These results will be used as a baseline for ongoing monitoring efforts at this protected area to assess the impacts of conservation efforts on the otter species present.
METHODS
Study Area
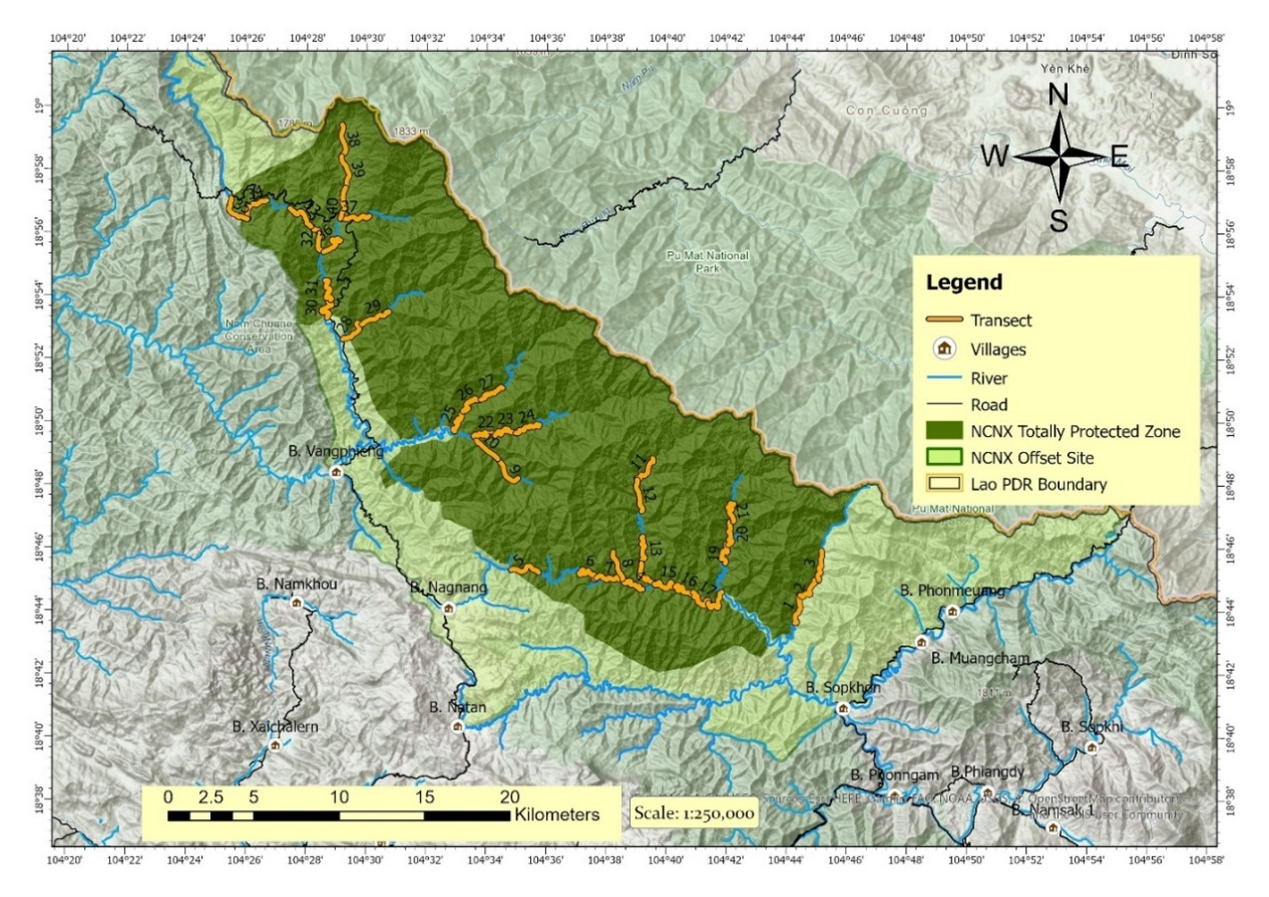
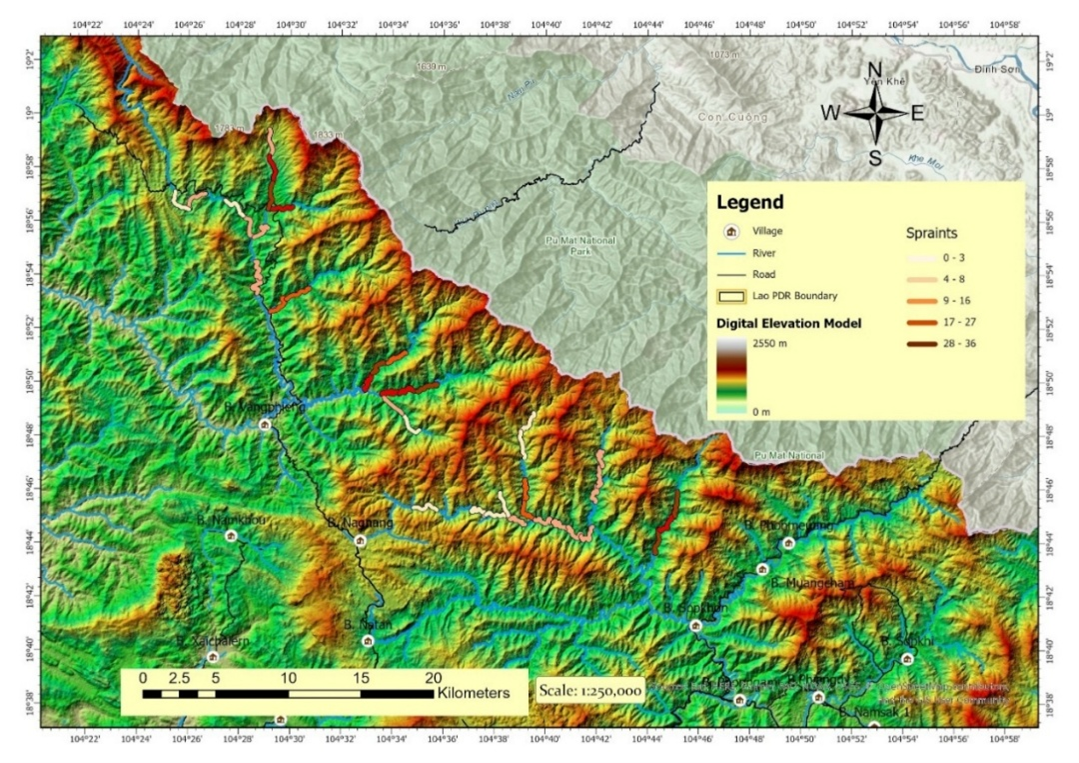
The Nam Chouane Nam Xang Biodiversity Offset Site (hereafter NCNX) is located in Bolikhamxay Province of Laos, in the northern Annamite Mountains (the ~1,000km-long mountain range which forms much of the border between Laos and Vietnam). The NCNX Offset Site is 810 km2 in size, with a 498 km2 Totally Protected Zone (TPZ), where access and use are restricted. The remainder is designated as a Controlled Use Zone (CUZ) meant for sustainable use by the site’s human communities. It sits on Laos’ border with Vietnam, forming a practical ecological continuation with one of Vietnam’s largest protected areas, Pu Mat National Park. The protected area is located between 104°23’E 19°12’N and 104°43’E 18°39’N and elevation ranges from 415 to 1,812 m above sea level (m.a.s.l.) (Figure 1).
Field Methodology
To determine otter species composition, distribution, and drivers of the distribution in NCNX, we installed camera traps, walked transects, and took measurements across the streams inside the NCNX TPZ. We used ArcGIS Pro to divide all streams inside the NCNX TPZ into 2 km segments. While spraint density is usually determined for transects of 400m-600m (Prenda and Granado-Lorencio, 1996; Jefferies et al., 2011; Prakash et al., 2012; Jayasurya et al., 2023), camera trap design for occupancy estimation and modelling typically aims to maintain distances of 2 kilometers between sampling stations or distances greater than the normal home range of the target species in order to avoid spatial autocorrelation (Abrams et al., 2018; MacKenzie et al., 2018). To have a common survey design for both metrics we opted for 2km-long transects. We randomly selected 40 of these segments distributed across all major river systems of the NCNX TPZ (Figure 1). Transect walks, stream measurements, and camera trap installations were all carried out during the driest time of the year in late March and early April of 2024.
Every transect was walked by the field surveyors, with team members covering both banks and recording all otter spraints. Additionally, team members recorded all human fires and camps in the transects. Each 2 km transect had three predetermined points spaced at distances of 0.5, 1.0, and 1.5 km from the start point of the transect. These evenly spaced points were used to make standardized stream measurements.
At each of these points, teams recorded (1) stream width, from the water’s edge on either side; (2) stream depth at five evenly spaced intervals across the width of the stream; (3) canopy cover, recorded as 0s and 1s directly above observer at the same five intervals where depth was recorded; (4) the predominant substrate of the river bed (mud, sand, pebbles, or rocks); and (5) whether the point was in a run, riffle, pool, or cascade. All length measurements were made with a 30-meter measuring tape. The tape was stretched across the river to measure width and to determine the 5 intervals for depth and canopy measurements. At each interval depth was measured with a bamboo staff, held against the outstretched tape after immersion to get an accurate measurement of depth. All binary canopy recordings were made using locally made canopy scopes, constructed from 50 mm PVC pipe and cotton string with crosshairs and a dangling weight (10 mm nut). These scopes allowed the researcher to pinpoint precisely 90° above their head while standing in the same location where depth was recorded. If the crosshairs (aligned with the weight) of the scope rested on sky, the value was recorded as 0, if the crosshairs rested on branches or leaves, the value was 1.
The teams installed a pair of camera traps (n=80) at their discretion somewhere between the first and last stream measurement points. These were spaced no further than 100 m apart and directed at unsubmerged rocks or logs with otter spraints or, if no spraints could be found, directed at unsubmerged rocks or logs where it was considered likely for otters to pass. Camera traps were a mixture of Scoutguard SG565FV with colour flash and Bushnell CORE™ Low Glow with infra-red flash. Teams returned an average of 62 days later (SD = 8.4) to collect these camera traps.
Data Analysis
We used camera trap results to identify otter species present in the landscape. Additionally, we had intended to use presence/non-detection data from these camera traps for occupancy modelling of the individual species (MacKenzie et al., 2018). Otter species were identified by the researchers and validated by an expert from the IUCN Otter Specialist Group. Independent events of otters and other fauna were determined at 30-minute intervals (≥30 min between photos determining a new event) and were recorded based on presence at the station. Individual camera traps in the pair and their results were not treated as independent. Photos were tagged and processed into record tables using camtrapR (Niedballa et al., 2016). Wild fauna were analyzed for a rate of detection (number of independent events of a species/total camera trap nights * 100) and a percent location (number of locations where a species was detected/total active locations * 100).
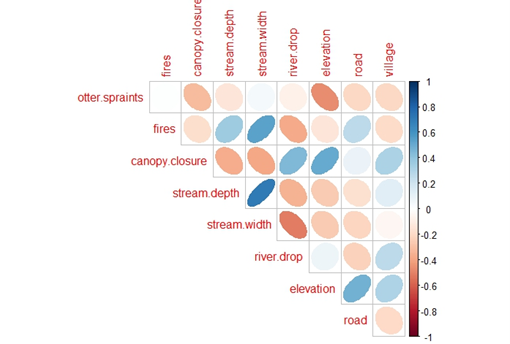
We used the transect spraint counts as a proxy of relative otter density (non-species-specific) and modeled this as a response variable in general linear models (GLMs) against natural and anthropogenic predictive variables. The natural covariates were: (1) average stream depth from the 15 measurements made at each site; (2) the cumulative count of ‘1’ values for canopy cover from 15 cross-section points at the site; (3) the average stream width from three locations at the site; (4) elevation in m.a.s.l. of the central stream measurement point; and (5) the elevation change between the start and end points of the transect. Elevation measurements were taken from a digital elevation model of Laos (Figure 6). The anthropogenic predictor variables were (1) count of fires and camps in the transect, (2) distance of the central stream measurement point to the nearest village, and (3) distance of the central stream measurement point to the nearest road.
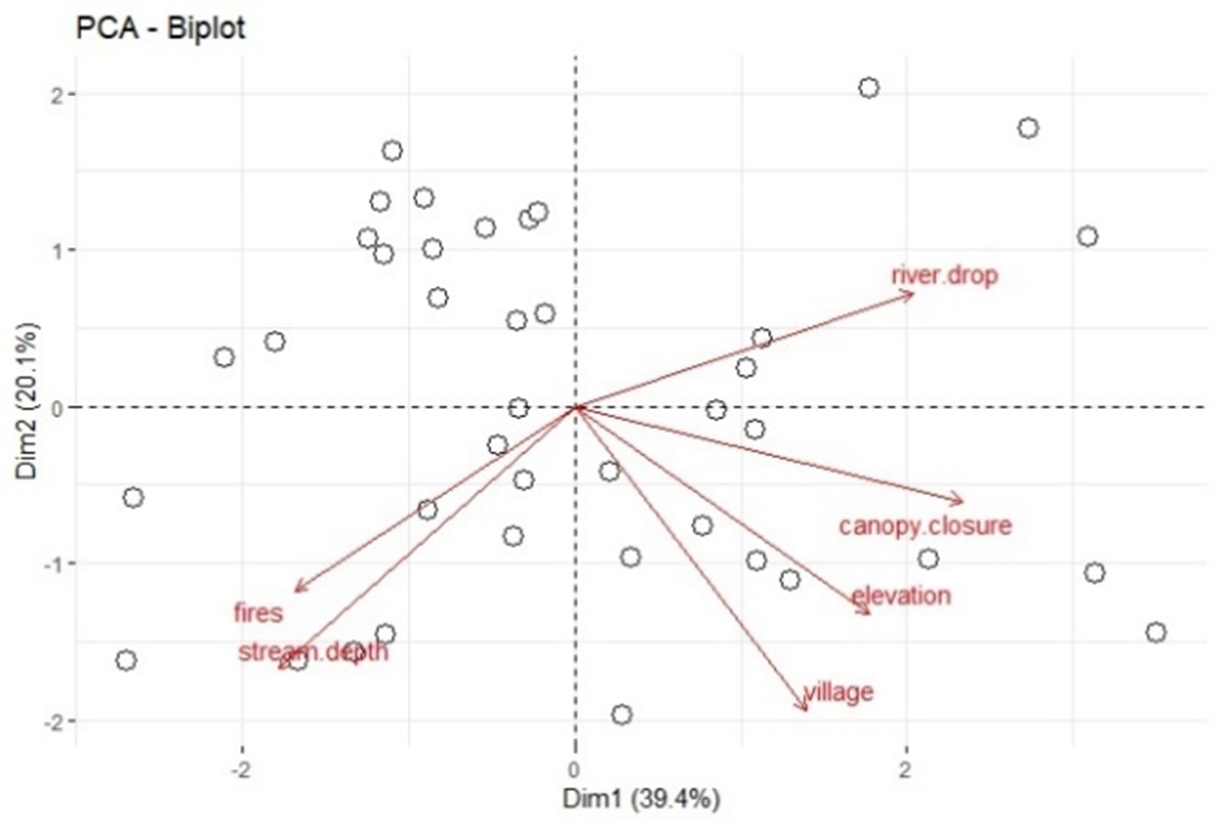
We ran a Principal Component Analysis (PCA) to reduce our predictors to four (n/10) and used these in linear models to predict otter spraint density. In a test of overdispersion (dividing the residual deviance of a full model by its degrees of freedom) otter spraint density was found to display overdispersion therefore, negative binomial GLMs were used to model otter spraint density against the predictive variables. Models included an intercept only model, a full model with all post-PCA covariates, a model for each of the post-PCA covariates, and a model for each possible pairwise combination of the four covariates. Akaike Information Criterion corrected (AICc) values were used for model selection, giving priority to (a) lowest AICc (within ∆2 AICc of the top-ranking model), (b) models with greater parsimony, and (c) models containing only covariates with p ≤ 0.05. Selected models were diagnosed in three steps: (1) examining the normality of the residuals (residuals displaying normal distribution and having mean values close to 0); (2) plotting of deviance residuals against fitted values (goodness of fit); and (3) using Cook’s Distance to identify influential points, then removing those points and re-running models to compare results. All models, model selection, model diagnostics, and other statistical tests were run in RStudio, R version 4.4.1 (R Core Team, 2022).
RESULTS
Camera Trap Detections
Of the 80 camera traps installed in the middle of the 2 km stream transects; nine were stolen, five had their memory cards stolen, and one had no photos due to technical issues. A total of 65 camera traps provided photos from 36 of the 40 survey sites over 2,245 active survey days (accumulation of active days at each station). From these, a total of 3,349 events of people and animals were detected. Of these events, 77 were otters; 27 small-clawed otter, 29 Eurasian otter, and 21 were unknown otter species but confirmed not to be smooth-coated otter (Table 1 and Figure 7).
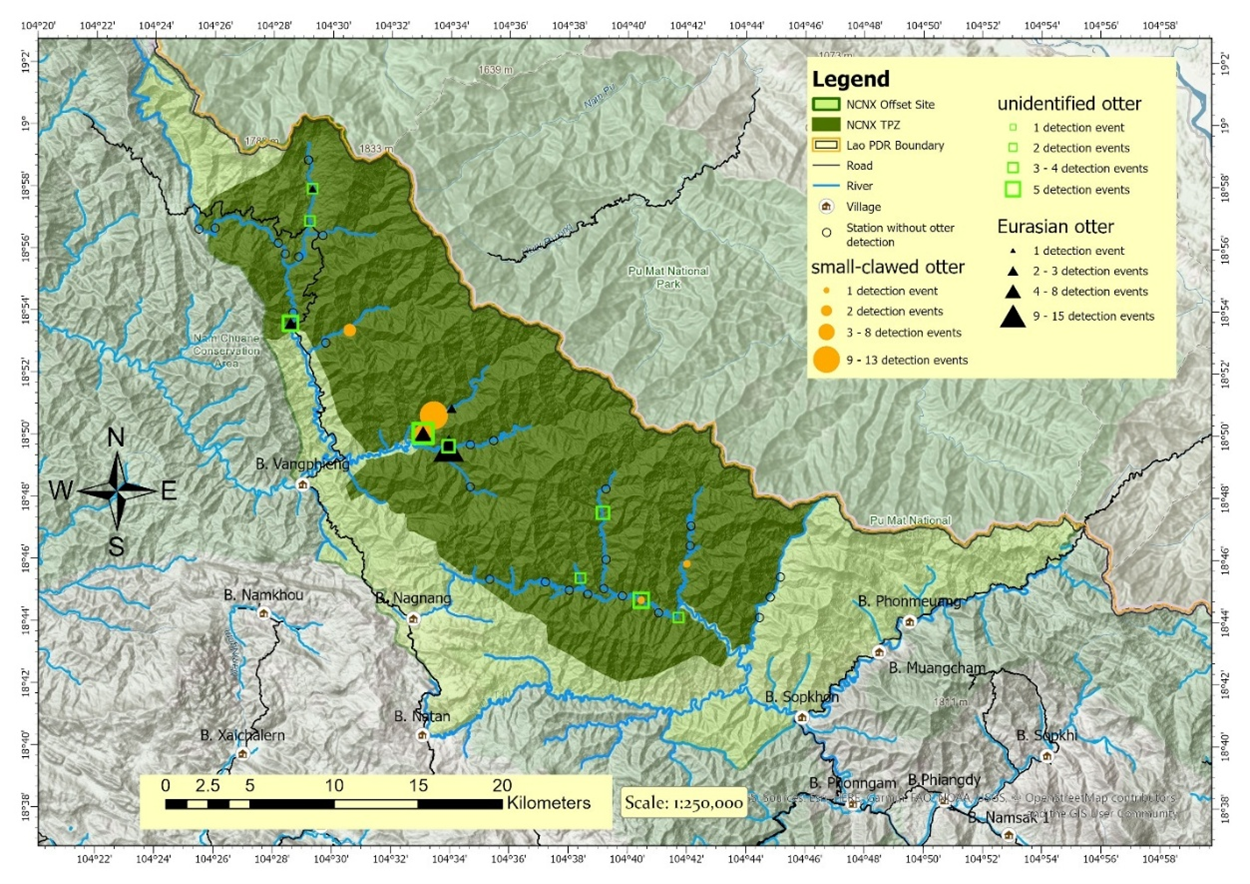
The detection rate of otters on the camera traps was lower than what we expected based on the spraint density - 1.2 detections per 100 camera trap nights for small-clawed otter,1.3 for Eurasian otter, and 0.9 for unidentified otter (Table 1). Considering the absence of otter detections from stretches of river with high spraint density and the fact that at most sites where otters were detected, they were only detected on one of the camera traps in the pair, we consider the camera traps to be ineffective in detecting otters when present. This makes the otter presence or absence in the images a poor indication of occupancy. Therefore, we did not attempt otter occupancy models with the camera trap data
In addition to the two otter species, we detected another 47 species of native fauna, as well as several unidentified species, two species of domestic animals, and human beings (survey team members and non-team members) (Table 1).
Spraint Density
On the 40 2km-long transects, the number of otter spraints detected ranged from 0 (at three sites) to >30 (at four sites) (average=11.88 SD=10.77) and the number of human camps and fires ranged from 0 (at seven sites) to >20 (at three sites) (average=5.48 SD=7.3). In running our PCA for the predictor variables of spraint density, we narrowed the predictor variables to (1) average stream depth, (2) elevation, (3) transect elevation change, and (4) human camps and fires. Prior to the PCA, we removed average stream width due to high multicollinearity with human camps and fires and average stream depth. Distance to road was also removed due to non-normal distribution and because certain roads at the site are not currently in use and are therefore a poor proxy for human presence, thus skewing the data. The first two dimensions of our PCA (representing about 60% of the variation in the remaining predictors) indicated that distance to nearest village, canopy closure, and elevation were clustered. Distance to village and canopy closure were removed because, of these, elevation had the strongest correlation to spraint density (Figure 5). Although fires and camps and stream depth were also clustered, we kept fires and camps as we considered it our best direct proxy for human use, and we kept depth as we considered stream size to be potentially important and it no longer had any other variable to represent it.
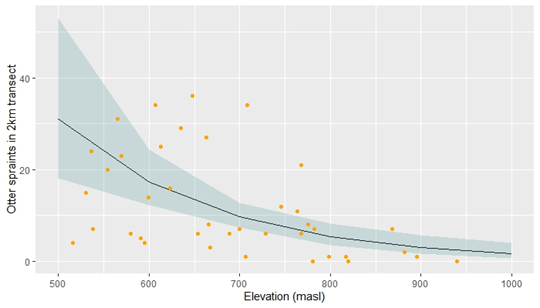
The top-ranking model (AICc=275.14, ∆AICc=0) was otter spraint density predicted by elevation alone, with a weak (beta-0.01) but significant (P<0.001) negative correlation. This model passed all model diagnoses. The only other model within ∆AICc≤2 was otter spraint density predicted by elevation and average stream depth (∆AICc=1.2) but stream depth was not significant (P=0.15) and had a negative correlation (beta-0.02).




DISCUSSION
We identified two species of otter in the NCNX TPZ, small-clawed and Eurasian, and observed spatial overlap of the two. There were no observations of smooth-coated otter, supporting our first hypothesis, that this species would be absent. Spraint density (which we used as a proxy for relative otter density) revealed a negative correlation of the relative density of these two species, combined, and elevation alone (with no support for other variables influencing otter density in the landscape). There has been debate over whether spraint density is an accurate reflection of otter density (Kruuk and Conroy, 1987; Mason and Macdonald, 1987), but the evidence following this debate has supported spraint density as a useful proxy for actual otter density (Guter et al., 2008; Lanszki et al., 2008). We were unable to test our second hypothesis that the two species would display niche segregation based on elevation and stream size due to the lack of reliable species-specific data. The camera trap data showed no evidence of one species being more dominant than the other or any unique spatial trends of either species.
Our data and models suggest no spatial avoidance of human activities in the landscape, i.e. hunting, fishing, non-timber forest product collection, and livestock shepherding; in contrast to patterns often observed for many wild mammals in Laos (Johnson et al., 2006; Hallam et al., 2016; Alexiou et al., 2025; White et al., 2025). Although intensive land use at high human densities has been associated with otter extirpation (Robitaille and Laurence, 2002), many studies have found that river otters are able to persist among human activities when habitat features remain (Sivasothi and Nor, 1994; Jefferies et al., 2011; Prakash et al., 2012; Akash et al., 2024). In Laos, where human caused mortality from hunting is the leading cause of faunal extirpation, the persistence of otters may be partly due to the fact that many Lao people do not like the taste of otter meat (J. White’s personal observation). This speculation is supported by the larger, non-otter-focused, camera trap survey of the site in the same year which revealed low detection rates of popular game species (ungulates, birds, and small carnivores) around the streams east of Ban Vangphieng (WCS Lao PDR, 2024); the same area which had the most otter detections by camera trap and streams all with relatively high spraint density. This suggests that, at this site, otters can avoid heavy mortality in the proximity of human hunting. However, there is an international demand for otters, particularly small-clawed otters (Gomez and Bouhuys, 2018), and they are found for sale in Lao markets (Schweikhard et al., 2019), therefore we can assume they are not entirely free from human persecution.
One reason for the observed negative correlation of spraint density and elevation may be that lower elevation correlates with greater river volume which is more closely related to the driving cause of differing otter densities. However, the inclusion of river depth as a covariate in our model comparison, and its insignificance as a predictor, rejects this hypothesis. Macdonald (1983) also found signs of Eurasian otter in Scotland to decrease with increasing altitude, concluding that food supplies were lower at higher elevations. Prenda and Granado-Lorencio (1996) found spraint density of Eurasian otter in Spain to be negatively correlated with elevation and found elevation to also be negatively correlated with fish size, concluding this was the causal factor. Similarly, White, McClean and Woodroffe (2003) found Eurasian otter density to be positively correlated with fish biomass. Sivasothi and Nor (1994) observed the presence of small-clawed otters in lowland wetlands, rice paddies, and mangroves. Dissimilar to these and our results, Akash et al. (2024) found no correlation of occupancy of small-clawed otter and elevation and Perinchery, Jathanna and Kumar (2011) found a positive correlation of small-clawed otter occupancy and elevation, finding the best habitat to be pools at higher elevations where crustacean biomass was believed to be greater.
We speculate that in NCNX, fish and crustacean biomass is greater at lower elevations and that this is driving the spatial patterns we observed. In addition to Prenda and Granado-Lorencio (1996), other studies have found correlations between elevation and fish biomass (Soo et al., 2021; Wanghe et al., 2024). While this is more likely to affect Eurasian otters than small-clawed otters, we consider it likely that there are similar patterns between elevation and crustacean biomass, which should be investigated further.
A limitation of this study was its restriction to the boundaries of the NCNX TPZ, applied in accordance with biological monitoring goals of the protected area. There are many reasons to believe these otter populations are not limited by these boundaries. These include the fact that; (1) we did not observe anything to suggest otters’ spatial avoidance of people, (2) we did observe a preference for rivers at lower elevations and the lowest elevation rivers of the landscape are outside the TPZ, (3) several studies suggest that river otters adapt to human activities when natural habitat features are preserved (Sivasothi and Nor, 1994; Jefferies et al., 2011; Prakash et al., 2012; Akash et al., 2024), and (4) the observations of otter spraints on stretches of river immediately adjacent to villages in the NCNX landscape (J. White’s personal observation). The restriction to the NCNX TPZ only served to reduce the study’s sample size and the variability of the predictor and response variables and thus it obfuscated drivers of the populations’ distribution in the landscape and factors to consider in their conservation strategy.
Another shortfall of the study was the failure to model the individual otter species due to this aspect of the study being reliant on occupancy modelling from camera trap data and the failure of the camera traps to consistently detect the species when present. The researchers’ speculation for possible reasons otters are not triggering the motion/thermal sensors of these cameras include the; (1) largely aquatic locomotion of the otters, (2) terrestrial locomotion of the otters over the rocks the camera traps were aimed at being camouflaged by the background movement of the water, (3) saturation of the otters’ pelts hiding the heat signatures of the animals, and (4) the low and narrow profile of otters not registering within the sensitivities of the cameras’ motion sensors.
While distinguishing between small-clawed and Eurasian otter spraints is reportedly possible based on the amount of crab remains (Kruuk, 2006; Prakash et al., 2012), we failed to observe any obvious differences, finding varying amounts of crab shells and fish bones in most spraints. We also found spraint sites with faeces of differing proportions of the two and a combination of well-formed faeces (suggesting Eurasian) and unformed faeces (suggesting small-clawed), supporting the theory that there is interspecific attraction of the two species to each other’s spraint sites (Kruuk et al., 1994) but generally making distinction and recording very difficult for the field teams. Due to this ambiguity, we considered the risk of misidentification to be high and did not ask field teams to record spraints by species.
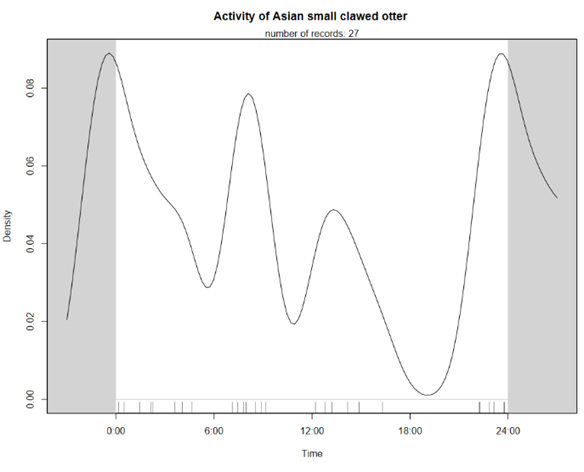
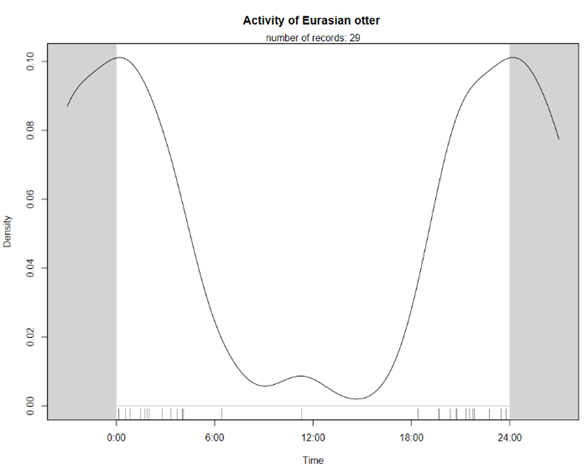
Although we failed to create occupancy models for individual species or examine evidence of niche segregation, it is apparent from the camera trap identifications that there is considerable spatial overlap of the two. This fits with the established understanding that these two similar species can niche segregate without spatial segregation due to their different diets (Kruuk et al., 1994). The temporal trends of the two species suggest more nocturnal behaviour of Eurasian otters but, due to the small sample size, this is likely coincidental and not strong evidence of temporal niche segregation.
RECOMMENDATIONS FOR OTTER CONSERVATION IN LAOS
Our recommendation for conservation managers tasked to keep otter populations in Laos viable, is to not limit their focus and actions to protected areas but to also consider the conservation value of rivers further downstream in human dominated landscapes still possessing riparian features and prey for the otter species in question. Not only does an exclusion of rivers outside of protected areas limit the available otter habitat, it is also likely to result in a focus on marginal otter habitats in the country, as most protected areas are located at the headwaters of the nation’s rivers and contain relatively little lower-elevation waterways. Actions in these unprotected stretches of rivers likely to be of great benefit to Lao otters include community fish conservation zones; preservation and enhancement of riparian vegetation, especially riparian forests (Jefferies et al., 2011); enforcement of laws protecting water resources from siltation and other forms of pollution; and stricter enforcement of laws against the use of explosives and electric current for fishing, practices disturbingly common in modern Laos (J. White’s personal observation).
Similarly, future research on otters should not be confined to protected areas and should include sampling locations in human-dominated landscapes. This should produce more varied and insightful data to better inform managers of the state of otter populations. Inclusion of larger rivers is also likely to increase researchers’ chances of encountering smooth-coated otter as this species is thought to rely more heavily on larger water bodies (Hussain and Choudhury, 1997; Anoop and Hussain, 2004). Researchers should also determine whether otters in Laos are persecuted for perceived competition over fish stocks. While we did not see evidence of spatial avoidance of human fishing activities in the study area, this could be an issue in other areas of the country, particularly in areas with greater human density.
Finally, we recommend that there be a formal examination of how camera traps could be used effectively for detecting otters. We found our camera trap results helpful in providing an inventory of the terrestrial species using the area’s streams, and if issues with sensors and detection could be resolved camera traps may also provide useful species-specific otter occupancy data. However, until these issues are resolved we do not recommend camera-trap reliant otter population surveys.
Acknowledgements: We would like to thank the biodiversity monitoring staff of the Nam Chouane Nam Xang Biodiversity Offset Site and the Nam Ngiep 1 Power Company for their work in collecting this data; Camille Coudrat for her review of our methods and reporting; and Nicole Duplaix for cross-validating the otter camera trap photos.
REFERENCES
Abrams, J.F., Axtner, J., Bhagwat, T., Mohamed, A., Nguyen, A., Niedballa, J, Sollmann, R., Tilker, A., and Wilting, A. (2018).Studying terrestrial mammals in tropical rainforests A user guide for camera-trapping and environmental DNA. Leibniz Institute for Zoo and Wildlife Research. ISBN: 978-3-9815637-6-4 https://www.izw-berlin.de/files/images/Wissenstransfer/UserGuide_online.pdf
Akash, M., Feldman, M., Ghose, A., and Tania, Z. (2024). Assessing habitat selection of the vulnerable Asian small clawed otters in an anthropized riparian forest of eastern Bangladesh. Mammal Research, 69: 101–114. https://doi.org/10.1007/s13364-023-00721-2
Alexiou, I., Coudrat, C., Niedballa, J., Wilting, A., and Tilker, A. (2025). Multi‐species occupancy modeling of ground‐dwelling mammals in central Laos: a case study for monitoring in tropical forests. Wildlife Biology, e01261. https://doi.org/10.1002/wlb3.01261
Anoop, K.R. and Hussain, S.A. (2004). Food and feeding habits of smooth‐coated otters (Lutra perspicillata) and their significance to the fish population of Kerala, India. Journal of Zoology, 266(1). https://doi.org/10.1017/S0952836905006540
CITES (2024). Appendices I, II and III to the Convention on International Trade in Endangered Species of Wild Fauna and Flora. Convention on International Trade in Endangered Species of Wild Fauna and Flora. https://cites.org/sites/default/files/eng/app/2024/E-Appendices-2024-05-25.pdf
Coudrat, C.N.Z., Choutipong, W., Sukmak, M., Sripiboon, A., and Klinsawat, W. (2022). Taxonomic status of otter species in Nakai‐Nam Theun National Park, Lao PDR, based on DNA evidence. Ecology and Evolution, 12(12). https://doi.org/10.1002/ece3.9601
Duckworth, W., Salter, R.E. and Khounboline, K. (1999).Wildlife in Lao PDR 1999 Status Report. Vientiane, Lao PDR. World Conservation Union : Wildlife Conservation Society : Centre for Protected Areas and Watershed Management. ISBN: 2-8317-0483-9 https://portals.iucn.org/library/sites/library/files/documents/2000-050.pdf
Duplaix, N. and Savage, M. (2018).The Global Otter Conservation Strategy (IUCN/SSC Otter Specialist Group). Strategy Doc. Salem, Oregon. ISBN: 978-0-692-04211-2 https://www.otterspecialistgroup.org/osg-newsite/wp-content/uploads/2018/12/IUCN-Otter-Report-DEC%2012%20final_small.pdf
Gomez, L. and Bouhuys, J. (2018).Illegal otter trade in Southeast Asia. TRAFFIC. ISBN: 978-983-3393-81-7 https://www.traffic.org/site/assets/files/5228/seasia-otter-report.pdf
Gomez, L., Leupen, B., Theng, M., Fernandez, K., and Savage, M. (2016). Illegal Otter Trade: An Analysis of Seizures in Selected Asian Countries (1980 - 2015). TRAFFIC. ISBN: 978-983-3393-49-7 https://www.traffic.org/publications/reports/illegal-otter-trade/
Guter, A., Dolev, A., Saltz, D., and Kronfeld-Schor, N. (2008). Using videotaping to validate the use of spraints as an index of Eurasian otter (Lutra lutra) activity. Ecological Indicators, 8(5): 462–465. https://doi.org/10.1016/j.ecolind.2007.04.009
Hallam, C.D., Johnson, A., O’Kelley, H., Saetuen, S., Thamsatith, T., O’Brien, T G., and Strindberg, S. (2016). Using occupancy-based surveys and multi-model inference to estimate abundance and distribution of crested gibbons (Nomascus spp.) in central Laos. American Journal of Primatology, 78(4): 462–472. https://doi.org/10.1002/ajp.22508
Hussain, S.A. and Choudhury, B.C. (1997). Distribution and status of the smooth-coated otter Lutra perspicillata in National Chambal Sanctuary, India. Biological Conservation,80(2): 199–206. https://doi.org/10.1016/S0006-3207(96)00033-X
Hussain, S.A., Gupta, S.K. and de Silva, P.K. (2011). Asian Small-Clawed Otter Aonyx cinereus (Illiger, 1815): A Review. IUCN Otter Specialist Group Bulletin, 28(2): 63–75. https://iucnosgbull.org/Volume28/Hussain_et_al_2011.html
Jayasurya, R., Moorthi, M., Sathishkumar, S., and Srimathi, R. (2023). Distribution, Habitat Selection and Diet of Smooth-Coated Otters (Lutrogale perspicillata) in the Kollidam and Thenpennai Rivers in South India. IUCN Otter Specialist Group Bulletin, 40(1): 3-15. https://www.iucnosgbull.org/Volume40/Jayasuryal_et_al_2023.html
Jefferies, M.R., Paukert, C.P., Whittier, J.B., Sandercock, B.K., and Gipson, P.S. (2011). Scale-dependent Factors Affecting North American River Otter Distribution in the Midwest. The American Midland Naturalist, 166(2): 177–193. https://doi.org/10.1674/0003-0031-166.1.177
Johnson, A., Vongkhamheng, C., Hedemark, M., and Saithongdam, T. (2006). Effects of human/carnivore conflict on tiger (Panthera tigris) and prey populations in Lao PDR. Animal Conservation, 9(4): 421–430. https://doi.org/10.1111/j.1469-1795.2006.00049.x
Khoo, M., Basak, S., Sivasothi, N., de Silva, P.K. & Reza Lubis, I. (2021). . The IUCN Red List of Threatened Species 2021: e.T12427A164579961. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T12427A164579961.en
Kruuk, H. (2006).Otters Ecology Behaviour and Conservation. Oxford University Press, Oxford, United Kingdom. ISBN: 0-19-856586-0 https://doi.org/10.1093/acprof:oso/9780198565871.001.0001
Kruuk, H. and Conroy, J.W.H. (1987). Surveying otter Lutra lutra populations: A discussion of problems with spraints. Biological Conservation, 41(3):179–183. https://doi.org/10.1016/0006-3207(87)90101-7
Kruuk, H., Kanchanasaka, B., O’Sullivan, S., and Wanghongsa, S. (1994). Niche separation in three sympatric otters Lutra perspicillata, L. lutra and Aonyx cinerea in Huai Kha Khaeng, Thailand. Biological Conservation, 69(1):115–120. https://doi.org/10.1016/0006-3207(94)90334-4
Lanszki, J., Hidas, A., Szentes, K., Révay, T., Lehoczky, I., and Weiss, S. (2008). Relative spraint density and genetic structure of otter (Lutra lutra) along the Drava River in Hungary. Mammalian Biology, 73(1): 40–47. https://doi.org/10.1016/j.mambio.2007.08.005
Loy, A., Kranz, A., Oleynikov, A., Roos, A., Savage, M. & Duplaix, N. (2022).Lutra lutra (amended version of 2021 assessment). The IUCN Red List of Threatened Species 2022: e.T12419A218069689. https://dx.doi.org/10.2305/IUCN.UK.2022-2.RLTS.T12419A218069689.en
Macdonald, S.M. (1983). The status of the otter (Lutra lutra) in the British Isles. Mammal Review, 13(1): 11–23. https://doi.org/10.1111/j.1365-2907.1983.tb00260.x
MacKenzie, D.L., Nichols, J.D., Royle, J.A., Pollock, K.H., Bailey, L.L., and Hines, J.E. (2018).Occupancy estimation and modeling: Inferring patterns and dynamics of species occurrence. Second Edition. Oxford Academic Press, Oxford. ISBN: 978-0-12-814691-0 https://doi.org/10.1016/C2012-0-01164-7
Mason, C.F. and Macdonald, S.M. (1987). The use of spraints for surveying otter Lutra lutra populations: An evaluation. Biological Conservation, 41(3): 167–177. https://doi.org/10.1016/0006-3207(87)90100-5
Ministry of Agriculture and Forestry (2023).Law on Wild Animal (Amended). Ministry of Agriculture and Forestry (MAF), Division of Forest Resource Conservation, Department of Forestry, Vientiane, Lao PDR.
Niedballa, J., Sollmann, R., Courtiol, A., Wilting, A., and Patrick, J. (2016). camtrapR: an R package for efficient camera trap data management. Methods in Ecology and Evolution, 7(12): 1457–1462. https://doi.org/10.1111/2041-210X.12600
Perinchery, A., Jathanna, D. and Kumar, A. (2011). Factors determining occupancy and habitat use by Asian small-clawed otters in the Western Ghats, India. Journal of Mammology, 92(4). https://doi.org/10.2307/23259879
Prakash, N., Mudappa, D., Ramen, T.R.S., and Kumar, A. (2012). Conservation of the Asian Small-Clawed Otter (Aonyx cinereus) in Human-Modified Landscapes, Western Ghats, India. Tropical Conservation Science, 5(1): 67. https://doi.org/10.1177/194008291200500107
Prenda, J. and Granado-Lorencio, C. (1996). The relative influence of riparian habitat structure and fish availability on otter Lutra lutra sprainting activity in a small Mediterranean catchment. Biological Conservation, 76(1): 9–15. https://doi.org/10.1016/0006-3207(95)00080-1
R Core Team (2022). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Robitaille, J.-F. and Laurence, S. (2002). Otter, Lutra lutra, occurrence in Europe and in France in relation to landscape characteristics. Animal Conservation, 5(4): 337–344. https://doi.org/10.1017/S1367943002004109
Schwartz, M.K., Luikart, G., and Waples, R.S. (2007). Genetic monitoring as a promising tool for conservation and management. Trends in Ecology & Evolution, 22(1): 25 - 33. https://doi.org/10.1016/j.tree.2006.08.009
Schweikhard, J., Kasper, K., Ebert, C., Lehmann, M., Ziegler, T., and Erbe, P. (2019). Investigations into the illegal wildlife trade in central Lao PDR. TRAFFIC Bulletin, 31(1): 19–25. https://www.researchgate.net/publication/334363049_Investigations_into_the_illegal_wildlife_trade_in_central_Lao_PDR
Sivasothi, N. and Nor, B.Hj.Md. (1994). A review of otters (Carnivora: Mustelidae: Lutrinae) in Malaysia and Singapore. Hydrobiologia, 285(1): 151–170. https://doi.org/10.1007/BF00005663
Soo, C.-L., Nyanti, L., Idris, N.E., Ling, T.Y., Sim, S.F., Grinang, J., Ganyai, T., and Lee, K.S.P. (2021). Fish biodiversity and assemblages along the altitudinal gradients of tropical mountainous forest streams. Scientific Reports, 11: 16922. https://doi.org/10.1038/s41598-021-96253-3
Wanghe, K., Ahmad, S., Zhou, X., Tian, F., Liu, S., Zhou, B., Nabi, G., Wang, G., Li, K., Jian, S., Jiang, H., Chen, S. Niu, Y., Khan, M.I., and Zhao, K. (2024). Spatially explicit estimation of freshwater fish stock biomass with limited data: A case study of an endangered endemic fish on the Tibetan Plateau, China. Science of The Total Environment, 912: 168717. https://doi.org/10.1016/j.scitotenv.2023.168717
WCS Lao PDR (2024).Camera Trap Survey in Nam Chouane – Nam Xang Offset Site of Nam Ngiep 1 Hydropower. WCS Lao PDR, Vientiane, Lao PDR.
White, J., Rasphone, A. and Syxaiyakhamthor, K. (2025). Assessing the Drivers of Distribution for a Diffuse and Cryptic Species over a Large and Rugged Landscape: Occupancy Modeling of the Critically Endangered Northern White-cheeked Gibbon [in submission].
White, P.C.L., McClean, C.J. and Woodroffe, G.L. (2003). Factors affecting the success of an otter (Lutra lutra) reinforcement programme, as identified by post-translocation monitoring. Biological Conservation, 112(3): 363–371. https://doi.org/10.1016/S0006-3207(02)00333-6
Wright, L., de Silva, P.K., Chan, B.P.L., Reza Lubis, I. & Basak, S. (2021). Aonyx cinereus. The IUCN Red List of Threatened Species 2021: e.T44166A164580923. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T44166A164580923.en
Résumé: La Densité des Loutres est corrélée à l’Altitude dans les Montagnes Annamites du Nord du Laos
Trois espèces de loutres ont été identifiées en République démocratique populaire du Laos (Laos) : la loutre à pelage lisse (Lutrogale perspicillata), la loutre eurasienne (Lutra lutra) et la loutre cendrée (Aonyx cinereus). Ces trois espèces ont connu un déclin démographique important et une réduction de leur aire de répartition à l’échelle mondiale et au Laos. Elles nécessitent donc des mesures de conservation ciblées pour assurer leur survie. Nous avons examiné la composition et la répartition de ces espèces dans une zone protégée du nord des montagnes Annamites au Laos. Nous avons utilisé des pièges photographiques et la densité de population (par approximation de la densité des épreintes) pour comparer l’importance de plusieurs variables naturelles et anthropiques dans l’estimation de la densité et de la répartition. Nous avons émis l’hypothèse que nous trouverions des traces de loutres eurasiennes et cendrées sur le site d’étude et qu’elles présenteraient des indices de ségrégation de niche en fonction de la taille et de l’altitude du cours d’eau, la loutre cendrée montrant une préférence pour les petits cours d’eau de haute altitude et la loutre eurasienne, l’inverse. Nous avons identifié deux espèces de loutres : la loutre cendrée et la loutre eurasienne, mais nous n’avons observé aucune preuve de ségrégation de niche entre elles. Nous avons constaté une corrélation négative significative entre la densité combinée de loutres (les deux espèces) et l’altitude, les deux espèces affichant une préférence pour les cours d’eau de basse altitude du site. De plus, nous n’avons trouvé aucune preuve d’évitement spatial des activités humaines. Cela suggère que les cours d’eau de plaine, y compris ceux situés hors des zones protégées du Laos, sont essentiels à la conservation des loutres et méritent des mesures de protection, tandis que les cours d’eau de haute altitude pourraient constituer un habitat plus marginal pour les loutres.
Revenez au dessus
Resumen: La Densidad de Nutrias se correlaciona con la Elevación en las Montañas Annamite Septentrionales de Laos
En la República Popular Democrática de Lao (Laos) se han identificado tres especies de nutria: la nutria lisa (Lutrogale perspicillata), la nutria Eurasiática (Lutra lutra), y la nutria de uñas pequeñas asiática (Aonyx cinereus). Las tres especies han experimentado declinaciones poblacionales y contracciones de la distribución significativas, globalmente y en Laos, y requieren acciones de conservación dirigidas para seguir siendo viables. Examinamos la composición específica y la distribución en un área protegida en las Montañas Annamite septentrionales de Laos. Utilizamos detecciones con cámaras-trampa y densidades poblaciones (como proxy de la densidad de fecas) para comparar la significancia de varias variables naturales y antropogénicas para predecir la densidad y la distribución. Hipotetizamos que en el sitio de estudio encontraríamos evidencia de nutria Eurasiática y nutria lisa, y que mostrarían signos de segregación de nichos en base al tamaño del río/arroyo y a la elevación -con la nutria de uñas pequeñas mostrando una preferencia por pequeños arroyos en elevaciones altas y la nutria Eurasiática, lo opuesto. Identificamos dos especies de nutria: la de uñas pequeñas y la Eurasiática, pero no observamos evidencia de segregación de nicho entre ellas. Encontramos una correlación negativa significativa de la densidad combinada de nutrias (ambas especies) con la elevación, ambas especies mostrando una preferencia por arroyos en elevaciones más bajas. Adicionalmente, no encontramos evidencia de evitación espacial de la actividad humana. Esto sugiere que los arroyos y ríos a bajas altitudes, incluyendo aquellos fuera de las áreas protegidas en Laos, son críticos para la conservación de nutrias y merecen esfuerzos de protección, mientras que los arroyos y ríos de altitudes elevadas pueden ser más marginales como hábitat de nutrias.
Vuelva a la tapa
ສະຫຼຸບສັງລວມ: ຄວາມໜາແໜ້ນຂອງ ນາກ ມີຄວາມສໍາພັນກັບລະດັບຄວາມສູງໃນເຂດພູດອຍທາງພາກເໜືອຂອງລາວ
ໃນ ສປປລາວ ໄດ້ມີການຄົ້ນພົບນາກທັງໝົດ 3 ຊະນິດຄື: ນາກໃຫຍ່ຂົມລຽບ (Lutrogale perspicillata ), ນາກໃຫຍ່ທໍາມະດາ(Lutra lutra) ແລະ ນາກນ້ອຍເລັeບສັ້ນ (Aonyx cinereus). ທັງສາມຊະນິດໄດ້ປະສົບກັບການຫຼຸດລົງຂອງຈໍານວນປະຊາກອນ ແລະ ພື້ນທີ່ອາໄສ ໃນທົ່ວໂລກ ແລະ ໃນລາວ ເຊິ່ງມີຄວາມຮຽກຮ້ອງໃຫ້ມີການອະນຸລັກຢ່າງຈິງຈັງ ເພື່ອຄວາມຄົງມີຢູ່ຂອງສັດເຫຼົ່ານີ້. ພວກເຮົາໄດ້ເຮັດການສໍາຫຼວດຊະນິດພັນ ແລະ ການກະຈາຍຂອງນາກ ຢູ່ປ່າສະຫງວນແຫ່ງໜຶ່ງໃນເຂດສາຍພູຫຼວງຕອນເໜືອຂອງລາວ. ພວກເຮົາໄດ້ນຳໃຊ້ຂໍ້ມູນການພົບເຫັນຈາກກ້ອງດັກຖ່າຍ ແລະ ຄວາມໜາແໜ້ມຂອງປະຊາກອນ (ໂດຍໃຊ້ມູນຂອງນາກເປັນຕົວແທນ) ເພື່ອປຽບທຽບໄນຍະສໍາຄັນຂອງປັດໄຈທາງທໍາມະຊາດ ແລະ ກິດຈະກໍາຂອງມະນຸດ ຕໍ່ຄວາມໜາແໜ້ມ ແລະ ກະຈາຍຂອງນາກ. ພວກເຮົາມີສົມມຸດຖາມວ່າ ຈະພົບເຫັນນາກໃຫຍ່ທໍາມະດາ ແລະ ນາກນ້ອຍເລັບສັ້ນໃນພື້ນທີ່ສຶກສາ ແລະ ພວກມັນຈະມີການແບ່ງແຍກພື້ນທີ່ຫາກິນຕາມຂະໜາດຂອງສາຍນໍ້າ ແລະ ລະດັບຄວາມສູງ ໂດຍຄາດວ່ານາກນ້ອຍເລັບສັ້ນຈະມັກອາໃສຢູ່ຕາມສາຍນໍ້ານ້ອຍໃນພື້ນທີ່ສູງ ແລະ ຈະກົງກັນຂ້າມກັນສຳລັບນາກໃຫຍ່ທໍາມະດາ. ຜົນການສຶກສາ ພວກເຮົາໄດ້ພົບການປະກົດມີສອງຊະນິດຄື ນາກນ້ອຍເລັບສັ້ນ ແລະ ນາກໃຫຍ່ທໍາມະດາ ໃນພື້ນທີ່ສຶກສາ ແຕ່ບໍ່ໄດ້ພົບຫຼັກຖານແບ່ງແຍກພື້ນທີ່ຫາກິນສະເພາະຂອງພວກມັນ. ພວກເຮົາໄດ້ພົບຫຼັກຖານຢ່າງມີໄນຍະສຳຄັນ ຂອງຄວາມສຳພັນທາງລົບລະຫວ່າງຄວາມໜາແໜ້ນລວມກັນ (ຂອງທັງສອງຊະນິດ) ກັບລະດັບຄວາມສູງ ໂດຍຫຼັກຖານບົ່ງຊີ້ວ່າທັງສອງຊະນິດມັກອາໄສຢູ່ຕາມສາຍນໍ້າໃນເຂດທີ່ມີລະດັບຄວາມສູງຕໍ່າ. ນອກຈາກນັ້ນ, ພວກເຮົາບໍ່ພົບເຫັນຫຼັກຖານຄວາມສໍາພັນທາງພື້ນທີ່ໃນການຫຼີກຫຼ່ຽງກິດຈະກໍາຂອງມະນຸດ ເຊິ່ງສິ່ງນີ້ບົ່ງບອກໄດ້ວ່າບັນດາຫ້ວຍນໍ້າທີ່ຢູ່ໃນເຂດພື້ນທີ່ຕໍ່າ ລວມທັງບັນດາສາຍນໍ້າທີ່ຢູ່ນອກເຂດອະນຸລັກຂອງລາວແມ່ນມີຄວາມສໍາຄັນຫຼາຍຕໍ່ກັບການອະນຸລັກນາກ ແລະ ຄວນໄດ້ຮັບການປົກປ້ອງຢ່າງຈິງຈັງ ໃນຂະນະທີ່ບັນດາສາຍນໍ້າທີ່ຢູ່ເຂດພື້ນທີ່ສູງອາດເປັນທີ່ຢູ່ອາໄສທີ່ເໝາະສົມໜ້ອຍກວ່າ
ກັບຄືນສູ່ຈຸດເລີ່ມຕົ້ນ


